Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2018) Volume 8, Issue 3
Herbal medication from natural resources plays an important role as antioxidant, antibacterial agent and demonstrating an inimitable property in wound curative rather than synthesized-toxic materials. The present investigation was designed to evaluate the potential healing efficacy of thyme (Thymus vulgaris) essential oil (TEO) oral adminstartion on topically treated wound with chitosan, TEO and their combination in vitro. Practically, effect of TEO oral administration on topically treated wounded rats in comparing to topical application of betadine for two weeks was carried out. Remarkably, treated group with TEO topically and orally exhibited the highest wound contractions among all treated groups. In Parallel, histopathological alteration was improved in TEO topically and orally applications whereas no degeneration, necrosis & inflammatory cells infiltration in skeletal muscle of wound with rejuvenation of hair follicles were observed. Wound healing efficiency was established in vivo and recommended as natural agent for wounds through protection of injury site from infections and inhibition of inflammatory cells as well as increasing connective tissue formation in repaired tissues. These favored results confirm that treated group with TEO topically combined with TEO orally have outstanding wound healing activity and need exploitation as an alternative source of natural wound agents for medical applications.
Keywords: Thymus vulgaris; Essential oil; Chitosan; Synergistic; Wound healing
As natural wound healing agents are not abundantly available, researchers are attentive to produce biocompatible and biodegradable wound healing agents that can increase healing efficiency. Currently, a significant number of superior drugs are identified and purified from natural sources such as plants containing bioactive substances against numerous diseases. As mentioned by WHO, approximately 80% of the world’s population remain depending on traditional medicines using natural sources [1]. Antimicrobial characteristics of herbal plants have been known and used since ancient times for food conservation and in the traditional medicine. Frequent studies have recognized that essential oils (EO) played a key-role and presented a great effect against a wide range of microbial species (including bacteria, fungi and candida) [2]. The antimicrobial properties of EO derived from numerous plants have been empirically recognized for centuries, but scientifically confirmed only recently [3]. Indeed, according to the Wound Healing Society, a wound defined as a physical injury or break of cellular, anatomic or functional of living tissue of the skin. Various mechanisms are involved in wound healing such as inflammation, coagulation, matrix synthesis and deposition, epithelization, angiogenesis, fibroplasia, remodeling and contraction [4]. Many people are suffering from chronic wounds globally because wounds represented active substrate for microbial infection. Wound healing material should have accomplished to provide an optimal environment around the wound and transporting bioactive elements or directly interacting with surrounding cells to facilitate/accelerate wound healing [5,6]. The appropriate wound healing process is a must for the rapid restoration of disrupted tissue as well as protecting organ’s failure and should prevent microbial infection, minimize the production of inflammatory mediators, eliminate blood and excess exudates, deliver or preserve moist environment, allow gaseous exchange (water vapor, oxygen), be thermally insulating, easily removable without causing trauma and comfortable, non-toxic and non-allergenic as well as cost effective [7]. Daily, betadine (Povidone Iodine) is used as an antiseptic material for wounds treatment. However, there is considerable controversy about using it being causes fibroblasts, keratinocytes devastation and inhibits lymphocyte and consequently causes delayed healing and subsequently pain as well as skin pigmentation and toxicity [8]. Bioactive compounds derived from natural resources (such as plants, microbial isolates, algae) have received a great interest due to the pharmacological activities, medicinal properties, low adverse effects and above all economic viability. On the other hand, chitosan (CH) has been proven to be a nontoxic, biodegradable, bio-functional, and biocompatible and has antimicrobial characteristics [9]. CH accelerate wound healing effectively because of its rapid dermal regeneration, accelerated wound healing properties besides its bacteriostatic effects [10]. The filmforming property of CH has found many applications in tissue culture and drug delivery, packaging by virtue of its mechanical strength and above all, rather slow biodegradation [11].
Thymus vulgaris is a well-known plant with aromatic features which is commonly used as a spice since ancient era. Thyme’s EO is enriched source of aromatic bioactive components such as thymol and carvacrol, which act considerable role as antimicrobial and antioxidative agents [12]. Burt et al. [13] stated that thyme EO contains mainly carvacrol, thymol, p-cymene and γ-terpinene. In addition, Braga et al. [14] confirmed that thymol have significant effects in regulating the inflammatory mechanism present in many infections, which is essential for proper wound healing. However, since inflammation causes many complications including wound dehiscence, impaired collagen synthesis and infection, thus anti-inflammatory effects of thymol would be a promising route naturally [15]. The commonly antimicrobial mechanism is considered as resulted by disturbing the function of cytoplasmic membrane, disrupting the active transport of nutrients to the cell membrane, and coagulation of microbial cell contents [16]. However, to the author’s knowledge, there are not any literatures available regarding clear application of thyme’s essential oil in the applications of wound healing. As mentioned, CH as wound healing agent was reviewed [10] but no available data are on the potential synergistic effects of CH and TEO mixed together. Therefore, the main aim of this research was to assess the potential wound healing efficacy of oral TEO administration and/or topical application of chitosan (CH), TEO and CH-TEOmix (mixture 1:1) in Wister rats. Basically, the antioxidant and antimicrobial efficiency of TEO have been carried out (first part of current study). Synergistic effects which may expected to improve the spectrum of medicinal activity comparing with both when used individuals have been investigated.
Essential oil
The high pure grade of dried herbs of thyme (T. vulgaris) essential oil (TEO) was obtained from the Fragrance and Extraction Factory, Sugar Industrial Integrated Company (SIIC), Cairo, Egypt using the hydro-distillation closed system.
Chitosan preparation
A 2.0% (w/v) chitosan solution was prepared by dissolving CH in 0.1% acetic acid solution. It was stirred till complete dissolving, then CH solution was placed for 24 h in a heater at 37°C under vacuum to favor acetic acid evaporation.
Biological activity tests
Animal model for wound healing: Thirty male adult Wistar rats weighing between 140-180 g were used in the current investigation. All experiments and investigation were approved by the Institutional Animal Ethics Committee (IAEC) of Qassim University, Saudi Arabia, regulated by the Saudi Law of Ethics of Research on Living Creatures and it’s Implementing Regulations (24/08/2010). Animals were individually housed in polypropylene cages in an air-conditioned room and kept in standard laboratory conditions under standard light and dark cycles (12 h light and 12 h dark) and maintained at an ambient temperature of 25 ± 1°C. The animals were fed normal pellet diet and water ad libitum. After 10 days of acclimatization, the animal’s boxes were divided into 6 equal groups (n=5/group), then rats were anaesthetized. Anesthesia was performed by intraperitoneal administration of ketamine (1.0 mL 10%) and xylazine (1.0 mL 2%) in the same syringe, and the back hairs were shaved by using a shaving machine. Consequently, one cm2 of full thickness of skin were removed using a sterile surgical blade from each side of each rat. The groups was treated as, G1: topically treated rats with 2% CH (TCH), G2: topically treated rats with 2% CH and 50 μl TEO orally administered (TCH+TEOO), G3: topically treated rats with TEO (TTEO), G4: topically treated rats with TEO and 50 μl TEO orally (TTEO+TEOO), G5: topically treated rats with TEO-CHmix (TCH-TEO mix) and G6: topically treated rats with TEO-CHmix (1:1) and 50 μl TEO as oral dose (TCH-TEOmix+TEOO). All previous groups were applied on left-side wounds while the right-side wounds were treated with betadine (Povidone-iodine-based medicated products as normal wound care, Gulf Care Factory, KSA). A sufficient amount (~100 μL) from CH, TEO or TEO-CHmix had been gently distributed over each wound day after day up to two weeks.
Wound healing assessment: The wound area of different treated groups was estimated by measuring the length and width of each wound using digital caliper two-day interval till 2 weeks or healing completion, the percentage of wound contraction (WC%) was calculated according to Balwada et al. [17] using the following equation:
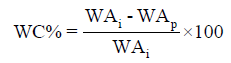 (1)
(1)
Where: WAi: Initial wound area and WAp: Periodically WA after 4, 6, 8, 10, 12 and 14 days of wound induction.
Histological examination: After decapitation, the cross-sectional full-thickness skin autopsy sample of healed wound were collected from all animal groups at the end of the experiment. The Autopsy samples were washed immediately with saline then fixed in 10% formal saline for 24 h. Washing was done in water then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56°C in hot air oven for 24 h. Paraffin bees wax tissue blocks were prepared for sectioning at 4 microns thickness by sledge microtome. The obtained tissue sections were collected on glass slides, deparaffinized and stained by hematoxylin and eosin stain for routine examination through the light electric microscope [18].
Statistical analysis: The statistical analysis was carried out using a SPSS program with analysis of variance (ANOVA) regarding to the experimental design under a significance level of 0.05 applying the Duncan test according to Steel et al. [19].
Wound healing activity of CH, TEO and CH-TEOmix In the in vitro phase of this study, results indicated that TEO exhibited high amount of total phenolic compounds (TPC). TEO exhibited high radical scavenging activity (RSA) toward DPPH, ABTS, linoleic acid deterioration and iron chelating activity. The TEO exhibit a high content of Thymol (41.04) as major compound over 14 identified components by GC-MS analysis followed by 1,8-Cineole (14.26), γ-Terpinene (12.06%), p-Cymene (10.50) and α-Terpinene (9.22). TEO exhibited antimicrobial activity in vitro and MIC noticed that TEO was efficiently affected pathogens in vitro. Investigated TEO or TEO-CHmix have strong antibacterial activity against many pathogenic bacteria and need exploitation as an alternative source of natural antibacterial and antioxidant agents (Accepted manuscript, [20]).
Effect of oral TEO administration and/or CH, TEO and TCH-TEO mix relative to betadine topical application on rat wound contarction percentage were illustrated in Table 1. All treated groups were found to have wound healing potential with different contarction percentages throughout the 2 weeks of experimental period. The means of wounds contractions of the whole treatments regardless curing period were 45.52 and 59.15% for topical B and CH (Group 1-P<0.05), respectively. While, means were increased up to 61.70 and 66.97% for topical B and CH given TEO orally (Group 2-P<0.05). Generally, histopathological alteration was improved in TEO orally treated group (Group 2 - Table 2) in topical B and CH wounds. The improvement grade of wound histopathological alteration (Table 2) were recorded to cover inflammatory cells in subcutaneous tissue (+), degeneration and necrosis in skeletal muscle (-) and inflammatory cells infiltration in skeletal muscle (-) in group 2 compared to group 1. A non-significant record between topical B and TEO (Group 3 - P>0.05) on wound contraction was detected in the current study (Table 1). The wound contraction enhanced for treated rats with TEO topically and orally (Group 4 - TTEO+TEOO) up to 68.69 and 75.77% for control wound (B) and treated one (P<0.05), respectively. Remarkably, TTEO+TEOO group exhibit the highest wound contractions records among all treated groups. In Parallel, histopathological alteration was improved in TEO orally treated group (Group 4) compared to group 3 in topical B and TTEO in underlying structure (No degeneration, necrosis & inflammatory cells infiltration in skeletal muscle) of wound with rejuvenation of hair follicles (Table 2). Obviously, moderate results have been obtained when rats treated with CH-TEOmix (Group 5) or CH-TEOmix administered TEO orally (Group 6). The wound contraction was 63.56 and 66.86% for topical B and CH-TEO mix, respectively, while it was 64.99 and 70.94% for topical B and CH-TEOmix given TEO orally, respectively (Table 1). The significant value of group 4 (TTEO+TEOO) for group mean were recorded the highest (72.23 ± 3.28) value relative to other groups in the current study. According to the mean of group significance, treatments were descending ordered as TTEO+TEOO (G. 4), TTEO (G. 3), TCH-TEOmix+TEOO (G. 6), TCHTEO mix (G. 5), TCH+TEOO (G. 2) and TCH (G. 1). These favored results confirm that treated group with TEO topically combined with TEO orally have outstanding wound healing activity.
| Group | Wound | Contarction percentage % / days | Mean of group | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 4th d | 6th d | 8th d | 10th d | 12th d | 14th d | Mean | |||
| G1 [TCH] |
B* | 26.11 ± 9.96aD | 27.17 ± 10.25aD | 52.73 ± 8.56aC | 64.48 ± 6.81aB | 90.29 ± 2.19aA | 94.14 ± 1.49aA | 59.15 ± 5.72a | 52.34 ± 4.96c |
| CH | -0.16 ± 13.13bE | 2.17 ± 18.53bE | 28.7 ± 12.76bD | 67.33 ± 4.39aC | 81.21 ± 1.88bB | 93.89 ± 1.48aA | 45.52 ± 8.01b | ||
| Mean | 12.97 ± 8.92E | 14.67 ± 10.82E | 40.72 ± 8.28D | 65.91 ± 3.85C | 85.75 ± 2.04B | 94.01 ± 0.99A | |||
| G2 [TCH+TEOO] |
B* | 22.64 ± 12.79aE | 42.93 ± 8.28aD | 62.21 ± 8.83aC | 84.70 ± 3.48aB | 92.33 ± 1.99aA | 97.03 ± 1.69aA | 66.97 ± 5.75a | 64.34 ± 4.01b |
| CH | 12.07 ± 4.45bF | 42.54 ± 7.77aE | 54.59 ± 6.11bD | 78.44 ± 5.36bC | 87.93 ± 2.44bB | 94.64 ± 2.18aA | 61.70 ± 5.65b | ||
| Mean | 17.36 ± 6.62F | 42.73 ± 5.35E | 58.40 ± 5.22D | 81.57 ± 3.19C | 90.13 ± 1.66B | 95.83 ± 1.36A | |||
| G3 [TTEO] |
B* | 27.71 ± 12.32bE | 40.06 ± 7.69aD | 61.08 ± 3.91bC | 82.57 ± 2.20bB | 92.61 ± 0.89bA | 98.01 ± 0.55aA | 67.01 ± 5.41a | 68.60 ± 3.81ab |
| TEO | 36.22 ± 11.83aD | 37.12 ± 7.66aD | 64.65 ± 6.82aC | 87.83 ± 3.09aB | 96.07 ± 1.25aA | 99.24 ± 0.42aA | 70.19 ± 5.43a | ||
| Mean | 31.96 ± 8.17E | 38.59 ± 5.14D | 62.87 ± 3.75C | 85.20 ± 1.99B | 94.34 ± 0.92A | 98.62 ± 0.39A | |||
| G4 [TTEO+TEOO] |
B* | 35.87 ± 7.99bE | 45.10 ± 8.00bD | 56.55 ± 5.27bC | 83.57 ± 4.58bB | 93.41 ± 1.88aA | 97.63 ± 1.48aA | 68.69 ± 4.90b | 72.23 ± 3.28a |
| TEO | 41.41 ± 9.33aE | 56.60 ± 5.16aD | 75.08 ± 6.13aC | 87.50 ± 3.70aB | 95.22 ± 1.83aA | 98.83 ± 0.34aA | 75.77 ± 4.33a | ||
| Mean | 38.64 ± 5.86E | 50.85 ± 4.88D | 65.82 ± 4.90C | 85.53 ± 2.85B | 94.32 ± 1.27A | 98.23 ± 0.74A | |||
| G5 [TCH- TEOmix] |
B* | 20.02 ± 5.87bF | 38.23 ± 11.63bE | 52.31 ± 13.20bD | 78.97 ± 5.37aC | 92.86 ± 2.97aB | 98.95 ± 0.42aA | 63.56 ± 6.13b | 65.21 ± 4.13b |
| TEOmix | 24.18 ± 12.65aE | 46.81 ± 8.94aD | 60.07 ± 10.22aC | 78.26 ± 2.87aB | 93.99 ± 1.45aA | 97.83 ± 0.79aA | 66.86 ± 5.63a | ||
| Mean | 22.10 ± 6.61E | 42.52 ± 7.06D | 56.19 ± 7.97C | 78.62 ± 2.87B | 93.43 ± 1.57A | 98.39 ± 0.46A | |||
| G6 [TCH-TEOmix +TEOO] | B* | 25.81 ± 7.81bF | 40.62 ± 5.81bE | 58.85 ± 7.95bD | 78.39 ± 3.46bC | 89.08 ± 2.80bB | 97.18 ± 1.14aA | 64.99 ± 5.19b | 67.97 ± 3.50ab |
| TEOmix | 38.83 ± 8.49aE | 45.06 ± 3.95aD | 62.48 ± 5.92aC | 87.30 ± 4.49aB | 94.39 ± 2.48aA | 97.61 ± 0.95aA | 70.94 ± 4.74a | ||
| Mean | 32.32 ± 5.85F | 42.84 ± 3.39E | 60.66 ± 4.71D | 82.85 ± 3.06C | 91.74 ± 1.97B | 97.39 ± 0.71A | |||
| Mean of period | 25.89 ± 3.02E | 38.7 ± 2.95D | 57.44 ± 2.59C | 79.94 ± 1.47B | 91.62 ± 0.74A | 97.08 ± 0.39A | |||
| Mean of wound | B* | Different treatments | |||||||
| 66.24 ± 2.48B | 87.20 ± 2.16A | ||||||||
B*: Topical betadine treatment,
a, b & c: There is no significant difference (P>0.05) between any two means, within the same column have the same superscript letter.
A & B: There is no significant difference (P>0.05) between any two means for the same attribute, within the same row have the same superscript letter.
Table 1: The wound contarction percentage of different experimental groups treated with CH, TEO and TCH+TEOmix (mean ± SE), (n=6).
| Groups | Treatment | Granulation tissue formation in the dermis | Inflammatory cells infiltration in subcutaneous tissue | Degeneration & necrosis in skeletal muscle | Inflammatory cells infiltration in skeletal muscle |
|---|---|---|---|---|---|
| Control | | | | | |
| G1 [TCH] |
B* | ++ | ++ | +++ | ++ |
| T** | ++ | | ++ | | |
| G2 [TCH+TEOO] |
B* | ++ | + | + | + |
| T** | ++ | + | | | |
| G3 [TTEO] |
B* | +++ | + | + | + |
| T** | ++ | | | | |
| G4 [TTEO+TEOO] |
B* | ++ | + | + | |
| T** | ++ | + | | | |
| G5 [TCH-TEOmix] |
B* | +++ | +++ | ++ | |
| T** | +++ | + | | | |
| G6 [TCH-TEOmix +TEOO] |
B* | + | +++ | ++ | ++ |
| T** | ++ | + | | |
Table 2: The severity of histopathological alteration in skin and underlying structure of different experimental groups treated with CH, TEO and TCH+TEOmix (n=6). B*: Topical betadine treatment, T**: Different topical treatment, +++ =Sever, ++ =Moderate, + =Mild, = Nil.
This result could be explained by studies of Braga et al. [14]. The former authors pointed that thymol as main component of TEO have bactericidal effect and can have helpful effects in regulating the inflammatory mechanism which is required for appropriate wound healing. Since inflammation causes many difficulties including infection, wound dehiscence and impaired collagen synthesis, anti-inflammatory effects of thymol would be promising material when thyme essential oil is used [15,21-23]. The commonly antimicrobial activity mechanism is considered as resulted by disturbing the function of the cytoplasmic membrane, disrupting the active transport of nutrients to the cell membrane, and coagulation of bacterial cell contents [16,24].
On contrary, treated group with chitosan exhibited negative significant differences (P<0.05) compared with B applications. Value of topical B was better curative agent than topical CH or CH given TEO orally, Table 1. This result was disagreeing with results of many researchers use chitosan as bioactive materials for wound healing due to using different concentration and/or unlike molecular weight chitosan [25,26]. Regardless of treatments types, the wound contractions were increased with time progression (Mean of period) as recorded in Table 1. As tailed in Table 1, a significant difference was found between topical B and different treated wound with CH, TEO and TCH-TEOmix as given TEO orally or not in general mean of wound contractions in whole experiment as 66.24 and 87.20%, respectively. Moreover, the wound treatment with B were recorded a massive granulation tissue formation, degeneration; necrosis in skeletal muscle and inflammatory cells infiltration in subcutaneous and skeletal muscle tissues (Table 2).
Similar findings have been suggesting that Hypericum perforatum oil and both sage and oregano EOs might be useful for the quick healing of severe and chronic wounds through protection of the injury site from infections and inhibition of the inflammatory cells as well as by increasing the connective tissue formation in the repaired tissue [27]. Also, Süntar et al. [28] revealed that essential oils obtained from the cones of Pinus pinea and P. halepensis displayed remarkable wound healing activity. The essential oil of Croton zehntneri and trans-anethole were improved cutaneous wound healing and demonstrated relevant therapeutic potential [29]. In addition, continuous application of essential oil of Lippia sidoides in adequate concentrations on cutaneous wounds increases inflammatory response without delay the lesions closure. The association of these results with antimicrobial action previously related to essential oil of L. sidoides allows its indication as an alternative therapeutic modality for topical treatment of infected cutaneous wound [30]. Our results are a new finding in the field of wound enhancer dressing using TEO and no previous studies highlighted this point so far even primary results were addressed [21,22].
Data in Table 2 and Figure 1A-X illustrated the severity of histopathological alteration in skin and underlying structure of different experimental groups treated with CH, TEO and TCH-TEOmix topically in both given TEO orally or not. In experimental groups, best remodeling, particularly, re epithelialization was observed in topical and oral TEO group with mild inflammatory cells infiltration in subcutaneous tissues relative to topical B treated wounds and other treated groups. Histopathological results in Table 2 are supported with the photographs of the tissues, which stained with H & E.
Figure 1: Histopathological view of wound healing and epidermal/dermal re-modeling. Normal skin before treatments: Skin of rat showing normal histological structure of epidermal and dermal (d) layers with hair follicles (h) and sebaceous (s) glands (1A) normal histological structure of the subcutaneous tissue with adipose (ad) structure and underlying skeletal muscles (m) (1B). [G. 1, TCH]: The skin showing wide area of granulation (g) tissue in the dermal layer with intact epidermis and lose of hair follicles and sebaceous gland in the focus area of granulation tissue (1C). Skin of betadine treated wounds showing wide area of granulation (g) tissue formation in dermis with intact epidermis (1D) massive inflammatory (m) cells infiltration in subcutaneous tissue and oedema (o) with degeneration and Zenker's necrosis (z) with the underlying skeletal muscle (1E). [G. 2, TCH+TEOO]: The skin screening intact epidermis with underlying granulation (g) tissue formation of fibrous tissue and newly formed blood capillaries in the dermis (1F). Skin of betadine treated showing wide area of granulation (g) tissue formation in dermis with intact epidermis (1G) massive inflammatory (m) cells infiltration in subcutaneous tissue with degeneration and Zenker's (z) necrosis and atrophy of the musculature (1H). [G 3, TTEO]: The skin showing hyalinization in granulation (hy) tissue at the dermal layer with intact epidermis and subcutaneous tissues (1I and 1J). Skin of betadine treated showing wide area of granulation (g) tissue in dermis with absence of sebaceous gland and hair follicle in it and intact epidermis (1K) few inflammatory (m) cells infiltration in subcutaneous tissue and in between the degenerated and atrophied skeletal (sm) bundles (1L). [G. 4, TTEO+TEOO]: The skin wide area of granulation (g) tissue formation in the dermis with intact epidermis (1M) focal inflammatory (m) cells aggregation in subcutaneous tissues underneath the granulation (g) tissue (1N). Skin of betadine treated showing focal wide area of granulation (g) tissue free from subcutaneous glands and hair follicles (1O) very few inflammatory cells infiltration (m) in subcutaneous tissue with degeneration (dm) in musculature (1P). [G. 5, TCH+TEOmix]: The skin showing focal area of granulation (g) tissue formation in the dermis with hyalinized (hy) hair follicle and focal few inflammatory (m) cells in subcutaneous tissue (1Q), 1R is magnification of 1Q to identify the focal inflammatory (m) cells in subcutaneous tissue (×40). Skin of betadine treated showing narrow area of hyalinized (hy) granulation tissue in dermis (1S) focal area of inflammatory (m) cells infiltration in subcutaneous tissue (1T). [G. 6, TCH+TEOmix+TEOO]: The skin screening wide area of granulation (g) tissue formation in dermis and extended deep in subcutaneous tissue (1U), 1V is magnification of (1U) to identify the inflammatory cells (m) infiltration in granulation tissue of subcutaneous area with atrophy of musculature (am) (×40). Skin of betadine treated showing wide area of granulation (g) tissue formation in the dermis with acanthosis (ac) in the epidermis (1W) focal few inflammatory (m) cells infiltration in subcutaneous tissue 1X (×16).
The TEO might possibly encourage the healing process at the initial steps of wound healing by acting as antimicrobial components [22]. In previous study of Süntar et al. [27] confirmed that wound healing and anti-inflammatory activities of olive oil extract of H. perforatum and its fractions exerted a significant effect on excision and incision wound models with a dose-dependent activity. This might be useful for the rapid healing of acute wounds through protection of the injury site from infections and inhibition of the inflammatory cells as well as by increasing the connective tissue formation in the repaired tissues. Similar findings were confirmed [2,3,21-23,31].
T. vulgaris EO topically and orally exhibited the highest wound contractions among all treated groups. The histopathological alteration was improved in TEO topically and orally applications whereas no degeneration, necrosis & inflammatory cells infiltration in skeletal muscle of wound with rejuvenation of hair follicles were observed. Wound healing efficiency was established in vivo and recommended as natural agent for wounds through protection of injury site from infections and inhibition of inflammatory cells as well as increasing connective tissue formation in repaired tissues. These favored results confirm that treated group with TEO topically combined with TEO orally have outstanding wound healing activity and need exploitation as an alternative source of natural wound agents for medical applications.
The authors are acknowledged the faculty of medicine, Qassim University for providing the Male adult Wistar rats for the whole experiment.
The authors declare no conflict of interest.