Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2014) Volume 4, Issue 2
This study aimed to characterize the different parts of Carica papaya (ripe and unripe papaya, leaves and seed), through their proximate composition, total antioxidant activity, and in vitro antiproliferative activities. Both macronutrients and micronutrients were determined using standard AOAC methods of analysis, while vitamin analyses were determined by high performance liquid chromatography (HPLC). Results showed that the ripe papaya had the highest antioxidant activity (84.04%) followed by unripe papaya (81.35%), leaves (78.03%) and the least was seed (75.35%). The phenolic content was in the order of leaves > unripe papaya > ripe papaya > seed. HPLC analysis showed that papaya leaves exhibited the highest ascorbic acid and β-carotene content (85.60 and 3.86 mg/100 g respectively), while the seeds had the highest Vitamin E content (4.09 mg/100 g). Results obtained from cytotoxic activities showed that MCF-7 (hormone dependent breast cancer) and MDA-MB-231 (non-hormone dependent breast cancer) cell cultures were significantly inhibited by the extract. The antioxidant and antiproliferative activities of different parts papaya extracts indicate that the consumption of the whole fruit, ripe and unripe papaya, leaves and the seed supplies the important quantities of numerous necessary nutrients for human diet which includes vitamins A, C and E.
HPLC: High Performance Liquid Chromatography; MCF-7: Hormone Dependent Breast Cancer; MDA-MB-231: Non- Hormone Dependent Breast Cancer; ROS: Reactive Oxygen Species; CaOV3: Ovarian cancer; HeLa: Cervical Cancer; AOAC: Association of Official Analytical Chemists; 3-(4,5-dimethylthiazol-2-yl)-2,5- diphenyltetrazolium bromide (MTT)
Natural antioxidants in vegetables and fruits, such as vitamins and polyphenols have been associated with the prevention of degenerative disease such as cancer and cardiovascular diseases [1]. The production of superoxide occurs continuously during normal aerobic metabolism [2]. Superoxide is a radical with an unpaired electron. Radicals usually are very reactive species, because electrons like to pair up to form stable two-electron bonds. Because of its radical character, superoxide is also called a «Reactive Oxygen Species» (ROS). The ROS formed can cause oxidative damage to various biological molecules like proteins, lipids and DNA. Therefore, there is an increasing interest in the antioxidant activity presents in the diet, since they play important roles in organism’s defense system against ROS [3].
As an important category of phytochemicals, phenolic compounds commonly present in plants have been considered to have high antioxidant ability and free radical scavenging capacity, with the mechanism of inhibiting the enzymes responsible for ROS production and reducing highly oxidised ROS [4], to exert chemopreventive, as well as protecting the human body against oxidative damage by free radicals [5].
Carica papaya belongs to the small family Caricaceae and is one of the non-seasonal and evergreen fruits in Malaysia. There is always a soft main trunk and tufted leaves at the top. Papaya varies in sizes, shape, color and taste. In Malaysia, the preference is for the redfleshed varieties namely Eksotika. The skin color of papaya is usually green when immature, changing to fully reddish-orange when fully ripened. The changed in outer color of the skin of fruit is an indicator of ripeness, and this change is considered mainly due to an increase in the carotene content and a decrease in chlorophyll. The color of papaya fruit flesh is determined largely by the presence of carotenoid pigments. Red-fleshed papaya fruit contain lycopene, whilst this pigment is absent from yellow-fleshed fruit [6]. The red-fleshed papaya has 63.5% of total carotenoids as lycopene which is absent in yellow-fleshed fruit [7]. Papaya contains a broad spectrum of phytochemicals including enzymes (in the latex), carotenoids (in fruits and seeds), alkaloids (in leaves), phenolics (in fruits, leaves, and shoots), and glucosinolates (in seeds and fruits).
Previous study have shown that papaya possesses anthelmintic, antiprotozoan, antibacterial, antifungal, antiviral, anti-inflammatory, antihypertensive, hypoglycemic and hypolipidemic, wound healing, antitumor, free-radical scavenging, antisickling, neuroprotective, diuretic, abortifacient, and antifertility activities [8]. In Jamaica, the ripe papaya is used as topical ulcer dressings to promote granulation, healing, and reducing odor in chronic skin ulcers [9]. In Nigeria, the green papaya is used for malaria, hypertension, diabetes mellitus, jaundice, intestinal helminthiasis. The leaves are used for colic, fever, beriberi, abortion, asthma in India [10], and cancer in Australia [11]. The milky juice (latex) is employed as styptic when applied as external applications to burns. People in Lao, Cambodia, and Vietnam use the latex to treat eczema and psoriasis [12]. The seeds have been used as vermifuge, thirst quencher, or pain alleviator [10].
Thus, the detailed study of the proximate composition of the ripe and unripe papaya, seed and leaves will contribute to the generation of data that can be used in tables of chemical composition of foods, as well as a better dietary guidance. In this sense, the aim of the present study was to determine the nutritional composition and antioxidant vitamins (Beta-carotene, α-tocopherol and ascorbic acid). Both macronutrients and micronutrients were determined using standard AOAC methods of analysis, while vitamin analysis was determined by HPLC. The second objective is to determine total phenolic and flavonoid content and antioxidant capacity using beta-carotene bleaching assay. An additional purpose was to evaluate the in vitro antiproliferative activity of different parts of papaya extracts on several cancer cell lines.
Chemicals
Methanol; chloroform; H2SO4; NaOH; boric acid; HCl, perchloric acid; anthrone reagent and petroleum ether (analytical grade) were all obtained from Fisher Scientific (Loughborough, UK). Beta-carotene; linoleic acid; Tween 20; α-tocopherol; and standard gallic acid, rutin, ascorbic acid (L-ascorbic acid, purity: 99%), alpha-tocopherol [(±)-alpha-tocopherol, purity: 95%) and beta-carotene (purity: 97%) were purchased from Sigma chemical Co. (St. Louis, USA). Folin- Ciocalteu reagent; sodium bicarbonate; aluminum chloride were purchased from Merck (Darmstadt, Germany). Methanol, acetonitrile and tetrahydrofuran were obtained from Merck KGaA (Darmstadt, Germany). All solvents were of HPLC grade.
CaOV3 (ovarian cancer); HeLa (cervical cancer); MDA-MB-231 (non-hormone dependent breast cancer); MCF-7, (hormone dependent breast cancer) and Chang liver cell (transformed normal cell line) were obtained from American Type Culture Collection (ATCC). Growth media; RPMI 1640 and phosphate buffer solution (PBS) were obtained from Sigma Chemical Co. (St Louis, USA). Fetal calf serum; gentamycin; doxorubicin hydrochloride and trypsin were obtained from PAA Laboratories, GmbH, Austria.
Plant materials and sample preparation
The papayas were bought from local market in Serdang, Malaysia. Throughout the experiments, the red-fleshed papaya was used. The maturity stages were visually defined according to the skin color as: Stage 0 - totally green; Stage 1 - yellow color that does not cover more than 15% of skin surface; Stage 2 - fruit with 16-25% of yellow skin; Stage 3 - fruit with 26-50% of yellow skin [13]. The fruits including the leaves, ripe and unripe fruit were washed under running tap water and separated into flesh and seed. The small cut piece of each sample was freeze-dried. The freeze-dried samples were ground into fine powder using a dry grinder. The ground samples were sieved to get uniform particle size, then kept in air tight containers and stored in a freezer (-20°C) until further analysis.
Chemical composition
Proximate analysis: The moisture was measured according to Ca 2d-25 AOCS [14]. The protein level was determined by the Kjeldahl method, according to Association of Official Analytical Chemists (AOAC) 984.13 [15]. The ash was determined by calcination at 550°C, according to Official Methods and Recommended Practices of the American Oil Chemists Society (AOCS) Ba 5a-49 [14]. Lipids were extracted in Soxhlet apparatus with petroleum ether at 40– 60°C, according to the method 3 75 Ai AOCS [14]. Total fibers were determined according to the method of Prosky et al. [16] proposed by AOAC 991.43 [15] and carbohydrates, by difference.
Minerals and vitamins analysis: Freeze dried samples were sent to the Malaysian Agricultural Research and Development Institute (MARDI) in Serdang Malaysia, for complete mineral analyses. Microwave digestion was used for sample decomposition to determine magnesium and iron using an inductively coupled plasma optical emission spectrophotometer (ICP-OES), method no. 984.27 [17]. The acid solution dissolved from ash residue was used for calcium, sodium and potassium analyses by flame atomic absorption spectrophotometer (AAS), method no. 975.03 [17]. Beta carotene was determined using the HPLC method according to method of Speek [18]. Analysis of vitamin E vitamin C was done following the method from Abdulnabi et al. (1997) [19].
Evaluation of the antioxidant capacity:
Extraction: The ground samples were extracted with 80% aqueous methanol (w/v, 1:25) at 200 rpm for 2 hour at ambient temperature with continuous stirring in a dark bottle using an orbital shaker (Heidolph Unimax 1010, Shcwabach, Germany). The mixture was filtered through a filter paper (Whatman No. 4). The obtained solutions were then used for total antioxidant activity, total phenolic and flavonoid content.
β-Carotene–linoleate bleaching assay: The antioxidant activities of different parts of papaya extract were assayed based on the β-carotene bleaching method [20-22]. The absorbance was read at 20 min intervals for 2 h at 470 nm. The antioxidant activity of extracts was based upon two different parameters, namely antioxidant activity (AA) and the oxidation rate ratio (ROR).
Antioxidant activity (AA) was expressed as percent of inhibition relative to the control by using the equation [21]:
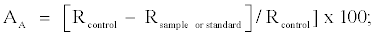
where Rcontrol and Rsample represent the bleaching rates of β-carotene without and with the addition of antioxidant, respectively.
Degradation rates (RD) were calculated according to first-order kinetics:
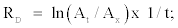
where ln is natural log, At is the initial absorbance at 470 nm at t = 0 and Ax is the absorbance at 470 nm at t = 20, 40, 60, 80, 100,120 min.
The oxidation rate ratio (ROR) was calculated as:
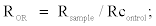
where Rsample and Rcontrol are as described earlier.
Total phenolic content: Total phenolic content was determined by spectrophotometry using Folin-Ciocalteu reagent method (Singleton and Rossi, 1965) with slight modification. A freeze-dried sample (200 mg) was extracted with 2 ml of 80% methanol at room temperature and then reacted with a 10-fold diluted Folin Ciocalteau reagent. Sodium carbonate at a concentration of 6% (w/v) was added, and the final volume was made up with deionized water. After incubation at room temperature in the dark for 2 h, the mixture’s absorbance was measured against the gallic acid standard at 725 nm. Total phenolic content were expressed as mg gallic acid equivalent (GAE)/100 g sample.
MTT reduction assay on human breast cancer (MCF-7 and MDA-MB-231) cells
The MTT assay is a colorimetric assay for assessing cell viability. Enzyme called NAD(P)H-dependent cellular oxidoreductase may reflect the number of viable cells present. These enzymes are capable of reducing the tetrazolium dye MTT 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyltetrazolium bromide to its insoluble formazan, which has a purple color. Cell lines, MCF-7 cells and MDA-MB-231 cells were obtained from the American Type Culture Collection (Rockville, MD) and were maintained in T75 flasks. Stock cultures were grown in a medium containing 5 ml RPMI 1640 (GIBCO BRL) supplemented with 5% fetal bovine serum. Gentamicin (50 μg/ml) was added to the experimental cultures. The cells were seeded in 96-well plates (100 μl cells well-1) and exposed to different sample concentrations in DMSO/ RPMI (3.125, 6.25, 12.5, 25, 50, 100 and 200 μg/ml) at 37°C with 5% CO2 for 72 h. After incubation, the MTT solution was added and the plates were incubated again for 4 h. The MTT solution was removed and the formazan product was fixed using 10% DMSO plus 90% isopropanol. Absorbance was measured at 570 nm using a plate reader (Lucy-1, Anthos). The viability was determined based on a comparison with untreated cells. Doxorubicin hydrochloride (0.78 to 25 μg/ml) was used as the positive control.
Statistical analysis
All experiments were carried out in 3 replicates and represented as mean ± standard error of mean (S.E.M). The SPSS version 20.0 was used for the data analysis. Statistically significant differences between groups were calculated by applying the one-way analysis of variance (ANOVA) for unpaired observations between controls and experimental samples. The Duncan test was used for multiple comparisons, and level of statistical significance was set at p ≤ 0.05.
Proximate composition
Table 1 presents the proximate composition of moisture contents in different parts of papaya extract. Ash, fat, crude protein and carbohydrate contents were recorded in grams per 100 g of dry material samples except for moisture content. Moisture value was significantly higher in unripe papaya (92.9 g/100 g) than the other parts (5.4 – 85.7 g/100 g). These values are similar to those found by Puwastien et al. [23] where ripe and unripe papaya was reported as 86.5 and 92.6 g/100 g respectively.
| Ripe papaya | Unripe papaya | Leaves | Seed | |
|---|---|---|---|---|
| Moisture | 85.7 ± 0.3b | 92.2 ± 0.01a | 75.4 ± 0.02c | 5.4 ± 0.05d |
| Crude protein | 6.1 ± 0.02d | 7.9 ± 0.03c | 33.4 ± 0.09a | 25.1 ± 0.08b |
| Ash | 5.8 ± 0.20d | 6.6 ± 0.04c | 11.4 ± 0.01a | 8.2 ± 0.08b |
| Total fat | 0.01 ± 0.05a | 0.00 ± 0.09a | 0.00 ± 0.01a | 0.00 ± 0.01a |
| Crude fibre | 7.6 ± 0.34d | 9.8 ± 0.09c | 14.1 ± 0.01b | 45.6 ± 0.05a |
| Carbohydrate | 70.7 ± 0.22a | 64.2 ± 0.06b | 38.4 ± 0.04c | 15.5 ± 0.03d |
Mean ± SD followed by different letter within a row are significantly different (P < 0.05).
Data are expressed as mean ± SD (n = 3).
Table 1: Proximate composition of different parts of papaya (g/100 g) of freeze dried samples.
The ash content estimated in leaves, seeds, unripe and ripe papaya were 11.4, 8.2, 6.6 and 5.8 g/100 g respectively. The results showed moderate value of ash content that suggest the different parts of papaya would provide essentials minerals. The leaves, seeds unripe and ripe papaya showed moderate crude protein contents of 33.4, 25.1, 7.9 and 6.1 g/100 g respectively. Crude protein contents of the ripe papaya increased by 32 and 50.3% correspondingly. Increase in crude protein during ripening in ripe papaya attributed to the increase to the conversion of enzymes or protein synthesis. It has been reported that protein synthesis is required for the ripening of the fruits [24]. The crude fat (0.00 g/100 g) was observed for the leaves, seeds and unripe papaya and (0.01 g/100 g) in ripe papaya. These results were significantly lower than earlier reported by Puwastien et al. [23]. The crude fiber of different parts of papaya were in the following order, seed > unripe papaya > leaves > ripe papaya. The total dietary fibre content of ripe papaya varies from 11.9 to 21.5 g/100 g dry matter [23]. The carbohydrate was present in moderate (15.5 g/100 g) to high (70.7 g/100 g) in the seed and ripe papaya respectively. However, previous study showed that the carbohydrate content was found to be decreased in the fully ripe papaya which is not in agreement with the present study [24].
Mineral contents
The mineral contents of the different parts of papaya are listed in Table 2. Significant differences in mineral content were observed between all the samples. Potassium was the predominant element among minerals analysed while iron were analyzed to be the lowest content. The leaves (3625.2 mg/ 100 g) had significantly higher potassium concentrations, while both ripe and unripe papaya was analyzed to be the lowest in iron content (1.8 mg/ 100 g). High amount of potassium in the body was reported to increase iron utilization [25] and beneficial to control hypertension through body fluid [26]. In the present study, high calcium (811.1 mg/100 g) and magnesium (564.9 mg/100 g) values were observed in the leaves while sodium was found to be high in the unripe papaya (84.8 mg/ 100 g). Sodium is the main cation outside cells and one of the primary electrolytes responsible for maintaining fluid balance. As recommended by Institute of Medicine [27] the adequate intake of sodium is 1500 mg/day for adults. This indicated that unripe papaya can contribute 5.65% of the recommended allowance.
| Minerals | Ripe papaya | Unripe papaya | Leaves | Seed |
|---|---|---|---|---|
| Ca | 120.1 ± 0.20d | 326.6 ± 0. 23c | 811.1 ± 0.04a | 681.4 ± 0.19b |
| Mg | 108.8 ± 0.22d | 172.5 ± 0.2c | 564.9 ± 0.1a | 423.5 ± 0. 4b |
| P | 2086.0 ± 0.15d | 2151.2 ± 0. 89b | 3625.2 ± 0.5a | 2115.7 ± 0.36c |
| Fe | 1.8 ± 0.46c | 1.8 ± 0.15c | 10.9 ± 0.24a | 5.8 ± 0.2b |
| Na | 20.6 ± 0.58d | 84.8 ± 0.41a | 24.4 ± 0.44b | 23.4 ± 0.23c |
Mean ± SD followed by different letter within a row are significantly different (P < 0.05).
Data are expressed as mean ± SD (n = 3).
Table 2: Mineral content of different parts of papaya (mg/100 g) of freeze dried samples.
Ascorbic acid analysis
The result obtained (Table 3) showed that papaya leaves exhibited the highest ascorbic acid content of 85.6 mg/100 g, followed by ripe papaya (45.8 mg/100 g), unripe papaya (37.8 mg/100 g) and the least was the seed (14.4 mg/100 g). There were significant difference (P<0.05) in ascorbic acid content of all sample extracts.
| Papaya plant Ascorbic acid content (mg/100g) | Ascorbic acid content (mg/100g) | β-carotene content (µg/100g) | Vitamin E content (mg/100g) |
|---|---|---|---|
| Ripe 45.75 ± 0.54b | 45.75 ± 0.54b | 520 ± 0.06b | 0.25 ± 0.01c |
| Unripe | 37.77 ± 0.28c | ND | ND |
| Seed | 14.44 ± 0.39d | 120 ± 0.39c | 4.09 ± 1.67a |
| Leaves | 85.60 ± 0.01a | 3860 ± 0.07a | 0.39 ± 0.07b |
Mean ± SD followed by different letter within a row are significantly different (P < 0.05).
Data are expressed as mean ± SD (n = 3).
Table 3: Ascorbic acid, β-carotene and vitamin E content of different parts of papaya extract (mg/100 g).
Compared to previous literature, the result obtained on L-ascorbic acid content observed in the present study was lower compared to others reported in the literature. Souza et al. [28] reported higher ascorbic acid content which were 90.7 mg/100 g and 71.3 mg/100 g in two different species of papaya which are Sunrise Solo 783 and Tainung 01 hybrid respectively. However Vinci et al. [29] reported ascorbic acid mean values of 54.0 mg/100g for ripe papaya and these findings were quite similar to the present study. Wall [30] suggested that papayas are a good supply of vitamin C and A. It ranks first among 13-17 fresh fruits for vitamin C content [31].
In the present study, ascorbic acid values were 10 folds higher in ripe papaya than those obtained for unripe papaya. Ascorbic acid content (AAC) increase during ripening of the present study was in agreement with quite a few of previous studies [32]. The AAC increased in ripe pericarp tissues of Capsicum annuum [33]. Howard et al. [34] have found that ascorbic acid content were 30% higher in red peppers compared to green peppers.
Beta-carotene analysis
The highest β-carotene content was found in young leaves (3860 μg/100 g DW) followed by ripe papaya (520 μg/100 DW), the seed (120 μg/100 g DW) and β-carotene was not detected in unripe papaya (Table 3). As reported in previous literature, total carotenoid content in papaya at different ripeness stages increased with the level of ripeness of the fruit [32]. Wenkam [35] reported that carotenoid content increased with maturation and ripeness. Andersson et al. [36] observed that the content of esterified carotenoids in cherries increased during ripening, which allows esterified carotenoids to integrate more quickly to the membranes, increasing the color of the fruit and its accumulation in chromoplasts [37]. The ripe papaya can be considered as a moderate source of provitamin A. They can range from 82 μg to 190 μg in 100 g fresh pulp. Souza et al. [28] reported the overall mean value for β-carotene observed was 370 μg in 100 g fresh ripe papaya.
Previous literature found that papaya contains an excellent amount of beta carotene. The mean of beta carotene content showed higher values than those previously reported, where papaya had 793.83 ± 5.47 μg /100 g edible portions [38]. Charoensiri [39] reported only 471 μg/100 g edible portions. Tee and Lim [40] in the study on carotenoid composition and content of Malaysian vegetables and fruits reported 228 μg /100 g edible portions of beta carotene in ripe papaya. Malaysian papaya has almost equivalent beta carotene value to that of Thai Food (1043 μg/100 g edible portions) [39].
Vitamin E analysis
A wide variation in vitamin E content was found in different parts of papaya extract, ranging from undetectable up to 4.09 mg/100 g (Table 3). The highest vitamin E content was found in papaya seed (4.09 mg/100 g) whereas the lowest were in the ripe papaya (0.25 mg/100 g). No peak of α-tocopherol was found in unripe papaya. The mean of vitamin E contents from this study did not completely agree with previous publications, especially for ripe papaya. Charoensiri et al. [39] reported non-detectable of α-tocopherol in ripe papaya. On the other hand, Monge-Rojas and Campos [41] detected 0.3 mg/100 g and 0.1 mg/100 g of α-tocopherol respectively in ripe papaya. As for β-carotene, its concentration increased in proportion to the ripeness and such of this change tendency does also agree in this study where α-tocopherol approaches its maximum level in the ripe papaya.
β-Carotene–linoleate bleaching assay
Beta-carotene bleaching method employs an emulsified lipid, which introduces a number of variables that influence the antioxidant activity of examined samples. The absorbance decreased rapidly in the samples without antioxidant whereas, in the presence of antioxidant, they retained their color, and thus absorb light for a longer time. It is probable that the antioxidant components in the papaya extracts can reduce the extent of beta carotene destruction by neutralizing the linoleate free radicals.
The comparable β-carotene bleaching rates of the control, α-tocopherol (standard) and methanolic extracts of different parts of papaya are shown in Figure 1. The β-carotene bleaching method is one of the most frequently applied methods for determining the total antioxidant property of the extracts. In the β-carotene bleaching assay, linoleic acid produces hydroperoxides as free radicals during incubation at 50°C and attacks the β-carotene molecules that cause reduction in the absorbance at 470 nm. The presence of antioxidants in the extract will minimize the oxidation of β-carotene by hydroperoxides. Hydroperoxides formed in this system will be neutralized by the antioxidants from the extracts. In this study, we evaluated the antioxidant activity of different parts of papaya extracts by the β-carotene– linoleate bleaching method because β-carotene shows strong biological activity and is a physiologically important compound [42].
Table 4 shows the mean antioxidant activity based on the β-carotene bleaching rate of the extracts of different parts of the papaya plant (ripe, unripe, leaves, seed). The extract with the lowest β-carotene degradation rate exhibited the highest antioxidant activity. As shown in Figure 1, all of the extracts had lower antioxidant activities than had standard (α-tocopherol). The highest antioxidant activity among the samples was observed in unripe papaya whereas seed had the lowest antioxidant activity. Result showed that there was considerably variation in the antioxidant activities where it ranges from the lowest of 58% to the highest of 91% where the orders of the antioxidant activity are as follow: α-tocopherol > unripe > leaves > ripe > seed.
There was a strong correlation between degradation rate and the bleaching of β-carotene. Table 4 shows that the extract with the lowest β-carotene degradation rate exhibited the highest antioxidant activity. Beta-carotene in the systems undergoes rapid discoloration in the absence of antioxidant and vice versa in its presence. The presence of different antioxidants can delay the extent of β-carotene bleaching by neutralizing the linoleate free radical and other free radicals formed in the system [43]. Thus, the degradation rate of β-carotene linoleate depends on the antioxidant activity of the extracts. Figure 1 showed the control had a substantial and rapid oxidation of β-carotene. Accordingly, the absorbance decreased rapidly in samples without antioxidant, while the sample extracts with the presence of antioxidant retained their absorbance for a longer time.
| Samples | AAa | RORb |
|---|---|---|
| Ripe | 84.04 ± 0.47a | 0.14 ± 0.01a |
| Unripe | 81.35 ± 1.25b | 0.18 ± 0.06b |
| Seed | 75.35 ± 0.96c | 0.25 ± 0.01c |
| Leaves | 78.03 ± 1.33c | 0.29 ± 0.01d |
Concentration sample was 0.04 mg/ml. Values are expressed as mean ± standard deviation (n = 3). Means with different letters within a column were significantly different at the level P < 0.05.
AAa Antioxidant activity index.
Table 4: Antioxidant activities of different parts of papaya extracts by β-carotene– linoleate bleaching method RORb Oxidation rate ratio.
Total phenolic content
Table 5 shows that the levels of total phenolic content (TPC) in the evaluated parts of the papaya plant varied significantly from 30.32 to 424.89 mg GAE /100 g of dry weight (DW). The leaves contained the highest phenolic content (424.89 ± 0.22), followed by the unripe papaya (339.91 ± 9.40), ripe papaya (272.66 ± 1.53) and the seed (30.32 ± 6.90).
| Samples | Total phenolicA |
|---|---|
| Ripe | 272.66 ± 1.53c |
| Unripe | 339.91 ± 9.40b |
| Seed | 30.32 ± 6.90d |
| Leaves | 424.89 ± 0.22a |
Concentration sample was 0.04 mg/ml. Values are expressed as mean ± standard
deviation (n = 3). Means with different letters within a column were significantly
different at the level P < 0.05. A Total phenolic was expressed as mg gallic acid
equivalent in 100 g of dry sample.
Table 5: Total phenolic content of different parts of papaya extracts.
The result indicates that the leaves contained high phenolic content that may provide good sources of dietary antioxidant. High phenolic content in papaya leaf extract were in agreement with previous study reported by Runnie et al. [44]. GC–MS quantitative analysis confirms that caffeic acid is the most abundant of all the identified compounds in papaya leaves and this compound might contribute to the total phenolic pool in the papaya leaves.
Several reports have conclusively shown close relationship between total phenolic content and antioxidative activity of the fruits and vegetables. Contribution of phenolic compounds is one of the mechanisms of the overall antioxidant activities. This mainly due to their redox properties involve in the plant material [45]. However, Pearson correlation showed that there was a weak positive relationship (r = 0.4713) found for antioxidant activity assayed by β-carotene bleaching assay with TPC. This was in agreement with Motalleb et al. [46] who also did not find any relationship between antioxidant activity and phenolic content in B. vulgaris fruit extract.
Total phenolic content
This is the first work exploring the antiproliferative effect of different parts of papaya extract against MDA-MB-231 (non-hormone dependent breast cancer) and MCF-7 (hormone dependent breast cancer). A range of different parts of papaya extract from (3.12 to 200 μg/ml) was used to investigate the decrease in cell viability against the selected cancer cell lines. Table 6 shows the concentration of different parts of papaya extract that inhibit 50% (IC50) of the cancer cells from proliferating. It was observed that only ripe papaya extract was active against MCF-7 (IC50 = 75.6 μg/ml). On the other hand, all the samples extracts capable to suppress the proliferation of MDA-MB-231 as shown in Table 6. Ripe papaya was found to cause 50% of cell death with the lowest IC50 of 49.3 μg/ml. Papaya leaves and seed exhibited low cytotoxicity with IC50 of 130.4 and 194.3 μg/ml. The entire sample extracts do not inhibited the proliferation of non-malignant Chang liver cells. This indicated that there was no discernable growth inhibition of the normal cell line and they were not cytotoxic to the normal cell. Lower cytotoxicity in normal cells compared to cancer cells is a prerequisite for any chemopreventive agent [11].
| Samples | Ripe | Unripe | Seed | Leaves | DOXb |
|---|---|---|---|---|---|
| MCF-7 | 75.6 | >200 | >200 | >200 | 25.0 |
| MDA-MB-231 | 49.3 | 75.1 | 194.3 | 130.4 | 3.1 |
aIC50 values (concentration of different part of papaya extracts that inhibit 50% of cell proliferation) were expressed as the mean ± standard deviation, in triplicate.
b Doxorubixin (positive control)
Table 6: IC50 of different parts of papaya extract against cancer cell lines.
It has been reported that more than 5000 compounds from papaya plant have been identified to be associated with the anticancer properties that include bioactive compounds such as phenolics, carotenoids and glucosinolates [47,48]. These bioactive act via multiple mechanisms such as cancer cell signaling, proliferation, apoptosis, migration, invasion, as well as angiogenesis and carcinogen elimination to exhibit in vitro anticancer activities [49]. Nakamura et al. [50] studied the apoptosis induction and inhibition of superoxide generation of n-hexane extract from papaya seed. The acute promyelotic leukemia HL-60 cells were exhibited by the papaya seed extract (IC50 = 10 μg/ mL). The experimental results suggested that these effects of papaya seed extract may be due to electrophilic compounds such as benzyl isothiocyanate. The effects of papaya flesh extracts on the viability of breast cancer cell line MCF-7 were examined concurrently with extracts from other fruits in two studies by Garcia-Solis et al. and Jayakumar et al. [51,52]. Garcia-Solis et al. [51] found that papaya had a significant inhibitory effect on breast cancer cell growth. The extracts from papaya flesh resulted in inhibition of proliferation of MCF-7 cells after a 72 h treatment.
This work reported that young leaves and ripe papaya were potent antioxidants while seed and unripe possessed comparatively moderate activities. Total phenolic content revealed that the extract of papaya leaves contain the highest amount of phenolic content. Taken together the antiproliferative study, these findings suggest that ripe papaya extract exhibited the highest antioxidant activity and potential in induction of apoptosis on the proliferation of MCF-7 and MDA-MB-231 cancer cell lines. However, further studies are needed to identify the active compound that confers the antioxidant and anticancer activity of ripe papaya extract.
>The authors are grateful to Universiti Putra Malaysia under RUGS initiative 6 grant scheme (Vote number: 9199607) for their financial support.