Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 3
Aim: The aim of the research was to investigate the dynamics of the parameters of carbohydrate and lipid metabolism, markers of associated inflammation in an induced metabolic syndrome (MetS) model, and to estimate the effectiveness of its treatment using a grape-derived stilbene concentrate (GDSC).
Methods: The research was performed on 40 white Wistar male rats of the SPF category aged 12 weeks. The MetS was induced using the fructose model (feeding the rats a diet with a 60% fructose content for 24 weeks). Rats with induced MetS were treated with GDSC, which was obtained by water-alcohol extraction of Vitis vinifera (Ressfood LLC, Russia).
Results: Induction of the experimental MetS was accompanied by the development of abdominal obesity, hyperglycemia, increased lipids levels, cardiomyopathy and damage of blood vessels. The expression of glucose transporter type 4 (GLUT4) and peroxisome proliferator-activated receptor-γ (PPAR-γ) had more expressive and early dynamics than biochemical shifts.
Conclusion: The development of the accompanying inflammatory reactions was confirmed by the increased expression of TLR4 and C-reactive protein as compared to control levels. The treatment of experimental MetS with GDSC prevented associated inflammatory reactions and protected the heart and blood vessels from damage.
Metabolic syndrome; Molecular markers; Associated inflammation; Grape-derived stilbene concentrate
The metabolic syndrome (MetS) is a cluster of the most dangerous heart attack risk factors: diabetes and raised fasting plasma glucose, abdominal obesity, high cholesterol and high blood pressure (The IDF Consensus Worldwide Definition of the Metabolic Syndrome, 2006). Currently, MetS is one of the most widespread forms of pathology across the globe. The scale of this problem has taken on an especially alarming character in industrialized countries, since a significant part of the population of these countries is exposed to a number of MetS risk factors: a sedentary lifestyle, a high-calorie and easily digestible diet, chronic stress, etc. For example, in the US, nearly 35% of adults, and 50% of those older than age 60, have MetS [1]. Pro-inflammatory, thrombogenic, and atherogenic potential of MetS increases the risk of life-threatening diseases such as diabetes mellitus type 2, severe cardiovascular pathology (myocardial infarction, ischemic stroke), and oncological diseases [2,3]. According to new data, MetS is a multifactorial pathology, which develops as a result of the polymorphism and disbalanced expression of the genes that regulate carbohydrate and lipid metabolism and immunological reactivity [4].
As mentioned above, one of the most important components of MetS is insulin resistance. Its occurrence is associated with the polymorphism of the genes responsible for the synthesis of peroxisome proliferator-activated receptor-γ (PPAR-γ) proteins. These proteins are involved in the transmission of the insulin signal [5,6]. Infringement of its transduction is realized at the postreceptor level. At the same time, hyperinsulinemia can compensate for a long time for disorders of the peripheral realization of insulin effects. As the syndrome progresses, β-cells are depleted and insulin resistance is complicated by insufficient insulin production [7]. This suggests that hyperglycemia, which is considered one of the main MetS criteria, occurs later than the molecular changes of MetS. According to the results of earlier investigation, tolllike receptors (TLR), which are the first line in immune defense reactions, play a significant role in the pathogenesis of MetS. They recognize non-specific bacterial antigens in infectious diseases, the lipopolysaccharides of intestinal bacteria with endointoxication, endogenous ligands (free fatty acids, triglycerides), and trigger reactions of innate and acquired immunity. Saturated fatty acids also can play the role of ligands of type TLR4 [8]. It has been shown that experimental obesity in mice, induced by feeding the mice a diet rich in fat, increases the expression of TLR2 and TLR4 in adipocytes and hepatocytes [9]. It has also been shown that, while mutation in the gene encoding TLR4 increases the risk of atherosclerosis and coronary heart disease, the concentration of circulating pro-inflammatory cytokines, fibrinogen, adhesins, C-reactive protein (CRP), and other inflammatory markers decreases. As a result, activation of TLR4 leads to subclinical systemic inflammation [10].
The results of recent investigations reveal several pathogenetic links between metabolic disorders associated with obesity and the development of low-grade inflammatory reactions. One of them is caused by the secretion of pro-inflammatory adipokines by adipocytes overloaded with triglycerides. It causes activation of immune cells with pro-inflammatory functions. In addition, the cells of the white adipose tissue produce pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor α (TNF-α), and monocyte chemoattractant protein-1 [11]. Moreover, lipid-overloaded hypertrophic adipocytes can cause associated inflammation in adipose tissue independently of adipocyte inflammation as a result of defects in glucose transporter type 4 (GLUT4) trafficking to the plasma membrane [12]. Regulation of GLUT4 gene expression within a very narrow range (2- to 3-fold) has a significant effect on whole-body glucose homeostasis, suggesting that a sort of homeostatic control of GLUT4 levels is an important adaptation to extreme physiological states or chronic adaptation to pathologic states, such as obesity [13].
The results of recent research prove that the relationship between metabolic disorders and the subclinical inflammatory response is bilateral. It has been shown that TLR4 signaling in the intestinal epithelium plays a key role in the regulation of metabolic syndrome through the regulation of microbial and host metabolic pathways [14]. The results of another investigation indicate that TLR4 may play an important role in the onset of steatosis, once its depletion enhances fatty acid oxidation in the liver of mice, preventing triglyceride accumulation [15].
The pathogenesis of MetS has a complex and multiunit nature with intricate relationships between different pathogenetic mechanisms. Accordingly, an effective treatment approach should have multidirectional therapeutic effects. Currently, the use of drugs based on plant extracts containing resveratrol, proanthocyanidins, stilbene, or flavonoids is considered one of the promising areas of pathogenetic therapy for MetS [16,17]. Grape-derived stilbene concentrate (GDSC) is a nowel polyphenol substance, full of stilbenes, especially trans-resveratrol and ε-viniferin. A number of studies have revealed the anti-inflammatory, antidiabetic, lipid-lowering, antioxidant and antiproliferative effect of transresvertarol [18-20]. One of the mechanisms of action of transresveratrol is the inhibition of pathological activation of the intracellular signaling cascade TLR 4/nuclear factor κB (NF-κB)/ Signal transducers and activators of transcription (STAT), and the reduction of cell apoptosis through the mechanism associated with the TLR4 signaling pathway/myeloid differentiation factor 88 (MγD88)/NF-κB [21].
The antidiabetic effect of polyphenols and their ability to increase the expression of the GLUT4 and PPAR-γ was also established, which leads to the restoration of insulin sensitivity and improved metabolic parameters [22].
The meta-analysis, summarized 16 controlled studies, was carried out to determine the association between the resveratrol intakes with metabolic parameters in MetS. Also combining the results of studies indicated significant effect of resveratrol on decreasing weight, triglycerides (TGs), systolic blood pressure and it can improve lipids level [23].
The aim of our study was to research the dynamics of the parameters of carbohydrate and lipid metabolism, the markers of associated inflammation in an induced MetS model, and to estimate the effectiveness of its treatment using a GDSC. Test meal that contain rice, meat, yogurt, spices etc.
Animal model and experimental design
The experiments were carried out on 40 white Wistar male rats of the SPF category (280-290 g, aged 12 weeks) (Laboratory Animal Nursery Pushchino, Russia). The animal use protocol was reviewed and approved by the Ethics Committee of V.I. Vernadsky Crimean Federal University (protocol No. 1, January 17, 2018) and followed the associated guidelines of the European Communities Council Directive of November 24, 1986 (86/609/ECC). Rats were housed in cages each containing five animals in a temperature-controlled room (20 ± 2°C) with a relative humidity of 60 ± 5% and a regulated light cycle (12 h light/dark) with free access to food and water. After 10 days of acclimation on laboratory chow, the rats were randomly allocated into four groups (n=10 per group): the control group of healthy animals with a control diet (Lab Rodent Diet 5001, 0.30% kcal from fructose), rats with experimentally induced MetS (highfructose diet for 24 weeks), rats with induced MetS treated with GDSC (from the 14th to the 24th week), and rats with induced MS treated with GDSC (from the 19th to the 24th week).
Diet
The metabolic syndrome was induced using the fructose model based on feeding the rats a diet with a 60% fructose content (Oriental Yeast Co., Ltd, Tokyo), which is much higher than of the standard solid feed-fructose-fed rats (FFR) (Research Diets, USA) [24,25]. Both diets contained sufficient vitamins and minerals to maintain rodent health. Except for biweekly overnight fasts, animals had ad libitum access to food and always had ad libitum access to water. The physiological state of the rats was monitored every day. Body weight and food intake were recorded every two weeks.
Experimental grape-derived stilbene concentrate
In our study, we used an experimental grape-derived stilbene concentrate, which contains catechin, resveratrol, proanthocyanidins, stilbenes, and other substances with multiple metabolic, antioxidant, and anti-inflammatory effects. The experimental grape-derived stilbene concentrate (GDSC) (Ressfood LLC, Russia), obtained by water-alcohol extraction of the Vitis vinifera grapevine, was used for treatment of MetS in one group starting from the 14th to the 24th week and in another group from the 19th to the 24th week of feeding. GDSC includes (g/L): gallic acid -0.158, (+)-D-cateсhin-0.379, (-)-epicatechin -0.446, trans-l -0.070, ε-viniferin -0.749, oligomeric proanthocyanidins -2.569, polymeric proanthocyanidins -17.329, non-identified stilbene -0.698, total stilbenes -1.517, and total phenols by highly effective liquid chromatography -22.40. GDSC has a similar polyphenols composition as “Enoant,” “Enoant Premium” and “Fenokor” [26], but additionally has trans-resveratrol, ε-viniferin and a lot of stilbenes. The safe effective dose of transresveratrol according to the Food and Drug Administration (FDA) and toxicology research is 2 mg/kg per day [27]. The dose of transresveratrol in GDSC was 0.070 g/L (0.07 mg/mL), so the total daily volume of GDSC for 280 g rats was 7 mL by oral tube, predissolved in water.
Sample collection and analysis
Once every 2 weeks, animals were subjected to an overnight fast before fasting blood glucose measurements (described below). Food was removed 1-2 h before the onset of the dark cycle, and returned after blood glucose measurements, approximately 12 h later. Fasting blood glucose was obtained via tail prick and measured using a Freestyle glucometer (Abbot Laboratories, Chicago, IL). Blood samples from animals were collected by tail vein blood sampling during experiments and by cardiac puncture at the end of the experiment. After standing at room temperature for 30 min, the blood was centrifuged at 4°C and the plasma recovered. Plasma aliquots for biochemical and lipidomic analyses were collected into Eppendorf tubes and then frozen using liquid nitrogen and stored at -80°C for further analysis. The concentrations of cholesterol and TGs were measured using commercially available kits (BioAssay Systems, Inc. USA).
After 24 weeks of dietary intervention and GDSC treatment, following a 12 h fasting period, all of the rats in each group were euthanized by an intraperitoneal injection of ketamine/xylazine and sacrificed by decapitation. After euthanasia, abdominal fat was extracted from the peritoneum in the mesentery of the small intestine and weighed (Adventurer AR 2140 electronic laboratory scale). Abdominal aortae and hearts were also harvested.
Inflammatory markers measurement
Enzyme-linked immunosorbent assays (ELISA) were used to measure the levels of TLR4, GLUT 4, PPAR-γ, and C-reactive protein in plasma using commercially available specific enzyme immunoassay ELISA kits (CUSABIO BIOTECH Co, Ltd) according to the manufacturer’s instructions. TLR4, GLUT4, and PPAR-γ are expressed as ng/mL, and C-reactive protein is expressed as mg/mL, with a detection range of 0.625 ng/mL-40 ng/mL, a sensitivity of 0.156 ng/mL, assay time of 1-5 h, sample volume of 50-100 μL, and a detection wavelength of 450 nm.
Heart and abdominal aorta histology
Morphological investigation of the heart and blood vessels was based on visible light and electron scanning microscopy. After 24 weeks of experimental intervention, hearts from all groups (n=4 per group) were fixed in 10% neutral buffered formalin, embedded in a single paraffin block, sectioned (cut as 6 μm), and stained with hematoxylin and eosin. Heart and abdominal aorta sections were examined by an independent experienced observer blinded to the treatment of the tissue. The morphological techniques used milling and the following equipment: LEEC LTD cutting station (Leica, Germany), LOGOS hybrid histological processor (Millestone, Italy), modular fill center Leica EG 1150 (Leica, Germany), automatic rotation microtome Leica RM 2255 (Leica, Germany), a Leica DM2000 laboratory microscope (Leica, Germany), Bond- Max immunohisto stainer (Leica, Germany), and an Aperio CS2 digital scanner (Leica, Germany). Scanning electron microscopy was carried out using a scanning electron microscope REM - 106 in high vacuum mode with an accelerating voltage of 20 kV. The pre-dried samples of heart were vacuum sprayed using a conductive coating in the form of a 20 nm layer of gold. All images were taken with lens N_Plan 40x or N Plan 10 SL and camera Leica DFC 495.
Statistical analysis was carried out using the Statistica 10.0 program using parametric (Student's T-test) and non-parametric (Wilcoxon’s W-test) tests or analysis of variance with post-tests for multiple comparisons, as indicated in the figure legends. Significance was assigned to p ≤ 0.05.
All measurements and studies were carried out using measuring instruments that passed metrological calibration and auxiliary equipment that was certified at the Center for Collective Use of Scientific Equipment (Molecular Biology) of the Medical Academy named after S.I. Georgievsky (structural unit) V.I. Vernadsky Crimean Federal University.
Our investigation demonstrated that experimental modeling of MetS is accompanied by the expression of metabolic disorders. The development of experimentally induced MetS leads to classic signs of MetS, including abdominal obesity, hyperglycemia, hypercholesterolemia, and hypertriglyceridemia. The main symptom of MetS-abdominal obesity was indicated. Abdominal fat in the MetS group was 2.5x that of the control group (p<0.001).
Treatment of MetS with the stilbene concentrate beginning in the 14th week caused the abdominal fat to decrease by 2.2x (p<0.001) that of the MetS group. Treatment of MetS with the stilbene concentrate beginning at the 19th week decreased abdominal fat by 40.0% compared to the amount of fat in the MetS group not treated with GDSC (Figure 1).
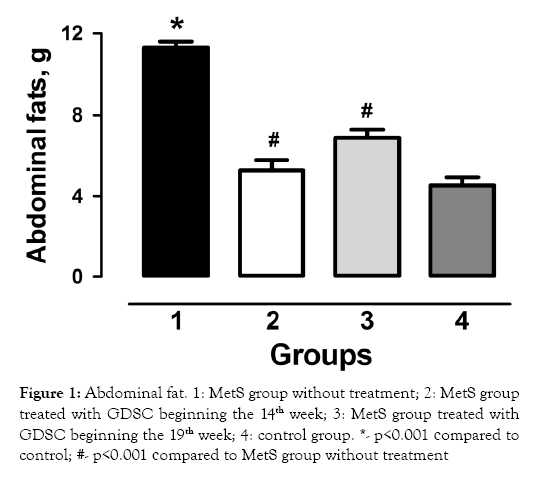
Figure 1: Abdominal fat. 1: MetS group without treatment; 2: MetS group treated with GDSC beginning the 14th week; 3: MetS group treated with GDSC beginning the 19th week; 4: control group. *- p<0.001 сompared to control; #- p<0.001 сompared to MetS group without treatment
We found that it took a long time for hyperglycemia to develop using this experimental MetS model in rats. Most of the time, the glucose level in the blood of the animals fed the high-sugar diet was even lower than the control values. The lowest value of this parameter was seen after 4 weeks of investigation. At that time point, it was 23.0% (p<0.01) lower compared to that of controls. It was only after the 18th week that the glucose level began to rise in comparison to that of the controls. By the 24th week, the average value of blood glucose in the induced MetS rats reached its maximum value (6.8 mmol/L), which was 17.2% (p<0.001) higher than that of the controls. At the same time, the average glucose level in the MetS group treated with the concentrate beginning the 14th week decreased to 5.0 mmol/L, which was 36.0% (p<0.001) lower than the blood glucose levels in the induced MetS group without treatment. Treatment of induced MetS rats with GDSC beginning the 19th week was less effective; the glucose level in that group decreased by 13.0% (p<0.05) compared to the blood glucose levels in the induced MetS group without treatment (Figure 2).
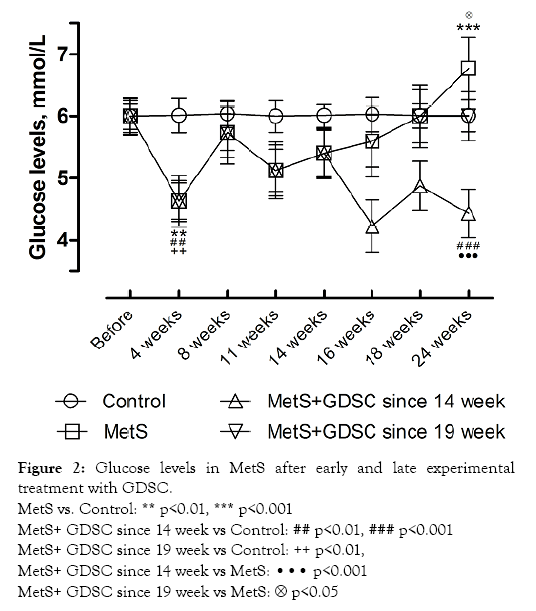
Figure 2: Glucose levels in MetS after early and late experimental treatment with GDSC.
MetS vs. Control: ** p<0.01, *** p<0.001
MetS+ GDSC since 14 week vs Control: ## p<0.01, ### p<0.001
MetS+ GDSC since 19 week vs Control: ++ p<0.01,
MetS+ GDSC since 14 week vs MetS: ··· p<0.001
MetS+ GDSC since 19 week vs MetS:  p<0.05
p<0.05
The results of these studies showed that the development of experimental MetS was accompanied with significant disorders of lipid metabolism. Thus, while the level of total cholesterol rose in a stable manner, it did not reach a statistically significant increase (15.8%; p<0.01) until the 24th week of fructose feeding. The experimental use of the stilbene concentrate prevented increases in the cholesterol level. In rats whose treatment with GDSC began in the 14th week, the cholesterol level was 28.0% (p<0.01) lower than in induced MetS rats without GDSC treatment; in rats whose GDSC treatment began in the 19th week, the cholesterol level was 17.3% (p<0.05) lower than in induced MetS rats without GDSC treatment.
One of the important criteria of MetS is the level of TGs in the blood. The results of our investigations showed that at 24 weeks of experimental development of MetS, the level of TGs increased by 38.0% (р<0.01) compared to levels in the controls. The levels of TGs in the groups with early and late treatment were 35.7% (p<0.001) and 34.7% (p<0.001) lower than in induced MetS rats without GDSC treatment, respectively (Figure 3).
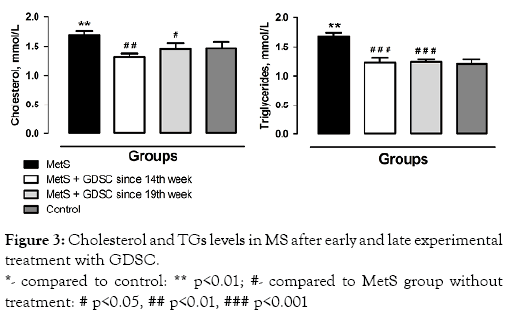
Figure 3: Cholesterol and TGs levels in MS after early and late experimental treatment with GDSC.
*- compared to control: ** p<0.01; #- compared to MetS group without treatment: # p<0.05, ## p<0.01, ### p<0.001
It is well known that the development of MetS is accompanied by heart disease. So the next stage of our research was to examine the morphology of the heart and blood vessels. It has been shown that MetS modeling causes damage to the heart and blood vessels, including stasis and perivascular edema, cleavage of cardiomyocyte bundles, endothelial damage, loosening of the aortic wall, destruction of the elastic skeleton, and soaking of the subendothelial intima layer with lipid inclusions (Figures 4, Figure 5 A1-2, B1-2).
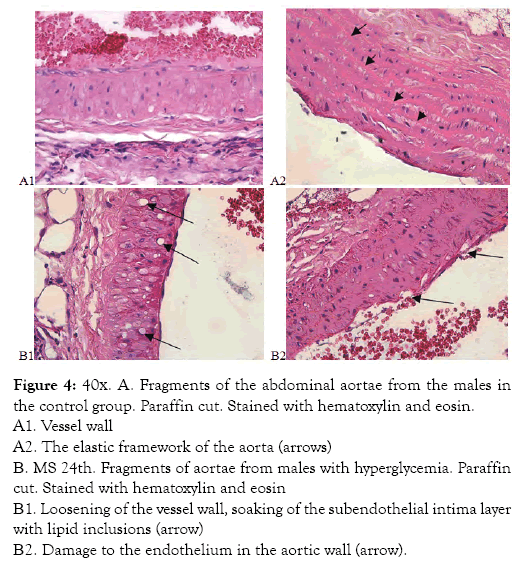
Figure 4: 40х. А. Fragments of the abdominal aortae from the males in the control group. Paraffin cut. Stained with hematoxylin and eosin.
A1. Vessel wall
A2. The elastic framework of the aorta (arrows)
В. MS 24th. Fragments of aortae from males with hyperglycemia. Paraffin cut. Stained with hematoxylin and eosin
В1. Loosening of the vessel wall, soaking of the subendothelial intima layer with lipid inclusions (arrow)
В2. Damage to the endothelium in the aortic wall (arrow).
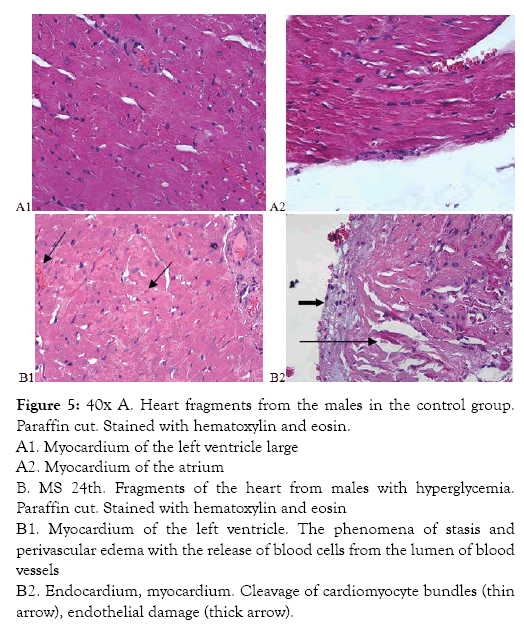
Figure 5: 40х A. Heart fragments from the males in the control group. Paraffin cut. Stained with hematoxylin and eosin.
A1. Myocardium of the left ventricle large
A2. Myocardium of the atrium
B. MS 24th. Fragments of the heart from males with hyperglycemia. Paraffin cut. Stained with hematoxylin and eosin
В1. Myocardium of the left ventricle. The phenomena of stasis and perivascular edema with the release of blood cells from the lumen of blood vessels
В2. Endocardium, myocardium. Cleavage of cardiomyocyte bundles (thin arrow), endothelial damage (thick arrow).
Treatment of experimental MetS with GDSC had a positive effect on the morphological alterations of the heart and aorta. This was confirmed by saved heart and vessels structure, saved cardiomyocyte myofibril striation, and abundant myocardial vascularization compared to MetS group without treatment (Figures 6A and 6B). Before the development of biochemical disorders, at the 14th week of feeding, the GLUT4 concentrations had already changed significantly-it was increased by 3.5x (p<0.001).
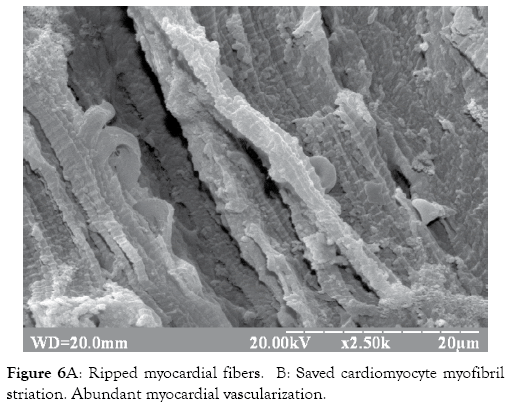
Figure 6A: Ripped myocardial fibers. B: Saved cardiomyocyte myofibril striation. Abundant myocardial vascularization.
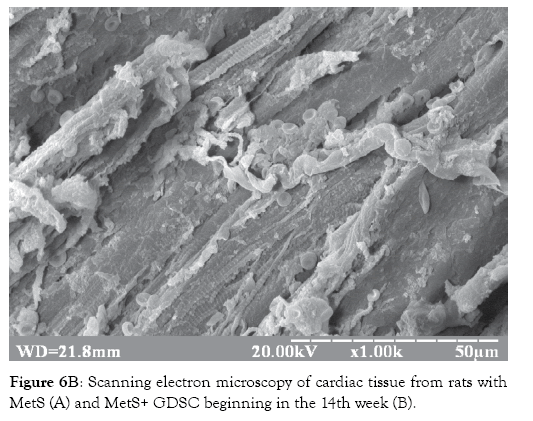
Figure 6B: Scanning electron microscopy of cardiac tissue from rats with MetS (A) and MetS+ GDSC beginning in the 14th week (B).
The experimental modeling of MetS by 24th week of feeding was accompanied with the increase of expression of the main transmembrane glucose transporter GLUT4 by more than 67x (p<0.001) compared to control values. GLUT4 levels increased by more than 125x in the group treated with GDSC beginning the 14th week compared to control values (p<0.001) and by 1.9x compared to the induced MetS group rats without GDSC treatment (p<0.01). In experimental MetS rats who received treatment with GDSC beginning the 19th week, the expression of GLUT4 decreased by 2.3x compared to levels in the MetS group (p<0.01) and by 4.4x compared to levels in the group treated with GDSC beginning the 14th week (p<0.001) (Figure 7).
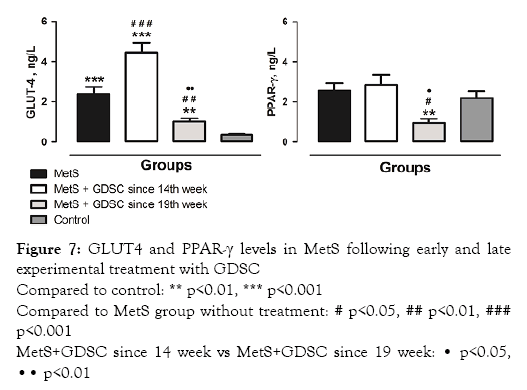
Figure 7: GLUT4 and PPAR-γ levels in MetS following early and late experimental treatment with GDSC
Compared to control: ** p<0.01, *** p<0.001
Compared to MetS group without treatment: # p<0.05, ## p<0.01, ### p<0.001
MetS+GDSC since 14 week vs MetS+GDSC since 19 week: • p<0.05,•• p<0.01
One of the key roles in maintaining energy homeostasis belongs to PPARs, a group of hormone-dependent nuclear transcription factors. The level of PPAR-γ receptor expression correlates reliably with the sensitivity of adipocytes to insulin. Induction of experimental MetS caused a compensatory increase in the expression of PPAR-γ receptors by 17%. Experimental treatment of MetS with a stilbene concentrate beginning in the 14th week of the experiment caused an increase in PPAR-γ receptors by 11.0% compared to those in the MetS group, while late onset treatment caused a decrease in the PPAR-γ level of more than 2x compared to the MetS group without GDSC treatment and by 3x compared to the group of MetS treated with the stilbene concentrate beginning the 14th week (p<0.05) (Figure 7).
Our research also revealed that induction of experimental MetS is accompanied by the development of an associated inflammatory response. This is based on the increase in the TLR4 concentration by 5.7x compared to that of controls (p<0.001). Experimental treatment of induced MetS with the stilbene concentrate beginning the 14th week caused a significant decrease in the TLR4 concentration by 2.8x compared to induced MetS group without treatment (p<0.001); the same results were received for the induced MetS group treated with the stilbene concentrate beginning the 19th week - decrease in the TLR4 concentration by 2.3x compared to induced MetS rats without treatment (p<0.001) (Figure 8).
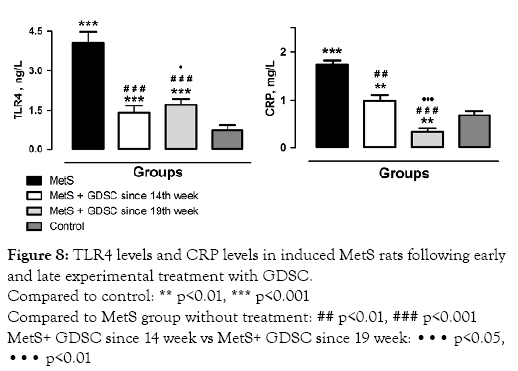
Figure 8: TLR4 levels and CRP levels in induced MetS rats following early and late experimental treatment with GDSC.
Compared to control: ** p<0.01, *** p<0.001
Compared to MetS group without treatment: ## p<0.01, ### p<0.001
MetS+ GDSC since 14 week vs MetS+ GDSC since 19 week: ••• p<0.05,••• p<0.01
C-reactive protein is one of the markers that indicates the development of a systemic inflammatory reaction in MetS. Our research showed that in the experimental model of MetS, this parameter increased by 2.5x (p<0.001) compared to control levels. In induced MetS rats treated with the stilbene concentrate beginning the 14th week, the C-reactive protein level was 76.0% (p<0.01) lower than in induced MetS rats without GDSC treatment. In experimental MetS rats treated with GDSC beginning the 19th week of the experiment, the C-reactive protein level decreased the most, by 5.4x compared to the induced MetS group without GDSC treatment (p<0.001) (Figure 8).
The results of our investigation demonstrate that the experimental model of MetS based on feeding rats solid food with a 60% fructose content is completely valid. It is proved by the expected trends in the levels of the indicators used as markers of MetS. At the same time, however, the characteristic shifts of important metabolic indicators traditionally used as the markers of MetSabdominal obesity, hyperglycemia, hypercholesterolemia and hypertriglyceridemia [28,29] showed convincing trends only by the 24th week. For example, most of the time of the glucose levels in the blood of animals with experimentally induced MetS was even lower than control values. It was only after the 18th week that the glucose level began to rise in comparison to those of the controls. This could be explained by the long-term compensation by means of hyperinsulinemia and overexpression of the main transmembrane glucose transporter GLUT4. It has been demonstrated that GLUT4 translocates to the cell surface in response to insulin-enhancing insulin sensitivity in an overexpression model [30]. PI3-K activation in insulin-stimulated GLUT4 plays a primary role along with Akt2 in contributing to insulin-mediated GLUT4 redistribution [31]. Again, only after the 18th week did the glucose level began to rise in comparison to that of the control, which in our opinion is evidence of the development of insulin resistance. Lipid metabolism disorders also showed characteristic dynamics during the several months of fructose feeding. The levels of total cholesterol and triglycerides reached mild but statistically significantly increased levels only by the 24th week of the experiment. In contrast, the concentration of GLUT4 had already changed by the 14th week.
In our studies we used GDSC as the experimental treatment for MetS: it is an experimental medicine, which according to information provided by the manufacturer, contains natural phytoalexins, proanthocyanidins, resveratrol, catechin, and other active ingredients. The results of our research showed that treatment with the stilbene concentrate has a significant positive effect on most of the metabolic, molecular, and morphological markers of experimental MetS.
Nevertheless, the effectiveness of treatment was different, depending on when it began. Of particular note was the difference in efficacy for metabolic markers. Thus, beginning the experimental treatment earlier (in the 14th week) caused a much more impressive decrease in the levels of glucose, cholesterol, and triglycerides compared with beginning the treatment in the 19th week.
Induction of experimental MetS was accompanied by a 67x increase in GLUT 4 levels (a 125x increase in the group treated with GDSC) compared to control values. It is suggested that so impressive an increase in the main transmembrane glucose transporter is a compensatory reaction, which helped to keep normal levels of glucose in the blood during the initial period of the experiment. The more expressive dynamics of GLUT4 under the influence of GDSC probably causes more insulin sensitivity and prevents glucose and lipid disorders.
Development of MetS caused changes in the concentration of PPAR-γ receptors, which enhanced the expression of the main transmembrane glucose transporter GLUT4. This was confirmed by the results of our study, which indicate a parallel increase in both PPAR-γ and GLUT4 in the experimental modeling of MetS coupled with early stilbene concentrate use beginning in the 14th week of research. In addition, PPAR-γ plays an important role in maintaining the energy homeostasis regulation of the differentiation of adipocytes and the accumulation of the lipids inside them [32,33].
Markers of the acute phase inflammatory reaction associated with the induction of MetS also showed significant elevation. Thus, the level of C-reactive protein in the induced MetS model was increased to more than twice its original level and the level of TLR4 expression showed 4-fold elevation. In this regard, the use of molecular markers of cellular responses and indicators of the associated inflammatory response for early diagnostics and assessment of severity of MetS seems appropriate.
The participation of systemic subclinical inflammation in the pathogenesis of MetS has been confirmed by numerous researchers [34-36]. A significant role in this process belongs to TLR. It has been experimentally proven [37,38] that the TLR4 in adipocytes can be activated by the action of free fatty acids, followed by the launch of NF-κB signaling pathways. This in turn causes the secretion of monocytic chemoattractant protein-1 (MCP-1), TNF-α, IL-1, IL-6, chemokines, and cytokines. Thus, it is logical to assume that TLR4 is the link between metabolic disorders and the development of subclinical systemic inflammation. The results of our study show that the development of MetS causes impressive increases in the molecular expression of TLR4. At the same time, the use of the GDSC prevents increases to the parameter and causes a significant decrease in the levels of the most dynamic and sensitive inflammatory marker. The same trend was shown by another classical marker for the intensity of systemic inflammatory reaction, C-reactive protein, but the decrease was not as dramatic. The positive influence of GDSC on the biochemical and molecular markers of experimental metabolic syndrome is confirmed by the results of the morphological investigation, which showed cardio and angioprotective effects in the MetS model for animals treated with the GDSC. Cardiac disadaptation is a result of a change in substrate availability to the cardiomyocyte. With the loss of insulindependent glucose flux, the predominant cardiomyocyte energy substrate would be fatty acids. The metabolic fate of glucose in the cardiomyocyte may contribute to the cytosolic redox state and cardiac damage [39].
In conclusion, molecular markers such as GLUT4, PPAR-γ, and TLR4 show more expressive dynamics than biochemical ones in metabolic syndrome, which supports the recommendation to use the listed molecular markers for early diagnosis and delivery of efficient treatment. The treatment of metabolic syndrome with the experimental grape-derived stilbene concentrate reduces the severity of metabolic disorders. The multi-vector therapeutic effect of GDSC reduces the severity of the inflammatory process associated with the metabolic syndrome. It is also worth noting that it is more effective when the treatment is begun as early as possible.
The authors declare no conflict of interests.
This work was partially supported by the V.I. Vernadsky Crimean Federal University Development Program for 2015-2024.
Citation: Petrenko V, Ganji V (2020) Pathogenetic Mechanisms of Metabolic Syndrome Development and its Correction with Grape-Derived Stilbene Concentrate. J Nutr Food Sci 10:769. doi: 10.35248/2155-9600.20.10.769.
Received: 02-Feb-2020 Accepted: 26-Feb-2020 Published: 04-Mar-2020 , DOI: 10.35248/2155-9600.20.10.1000769
Copyright: © 2020 Petrenko V, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.