Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 3
Objective: The aim of this work was to determine whether the changes in tryptophan-5-hydroxylase (TPH) 1 or 2 brain expression caused by intrauterine growth restriction (IUGR) are maintained or return to normal in the nutritionally-recovered undernourished offspring (UNDO) of rats.
Methods: The experimental procedure was: Wistar female rats were subjected to a protein-caloric-restricted diet during gestation. At birth, part of the newborn UNDO pups were fed by their own mothers (UNDO Group), and others were fed by normal control mothers to allow their nutritional recovery (NR Group); pups from normal control dams were fed by them (C Group). On days 1, 15 and 21, brainstem tissue, at the level of dorsal raphe nucleus (DRN), was obtained, in order to determine L-tryptophan (L-Trp), serotonin (5-HT) and TPH activity. In addition, TPH 1 or 2 expressions were determined using specific monoclonal antibodies by Western blot and by immunohistochemistry in neurons.
Results: In the UNDO group, an increase in L-Trp, 5-HT and TPH activity was confirmed. Both TPH isoforms were expressed since birth, with TPH1 expression being higher in the UNDO group. TPH2 activity was also elevated but was lower activity in comparison with controls. The NR group was able to physically recover to normality, but their neurochemical changes and elevated number of TPH1 neurons remained unchanged and did not return to control values. TPH2 neuronal number did return to normal control values.
Discussion: These results strongly suggest that the changes in the UNDO group may explain, together with a possible important Pet-1 molecular system participation, the chronic activation of the brain serotonin system in subjects that suffered IUGR due to undernourishment.
In-utero undernourishment; Serotonin neurons; Tryptophan-5-hydroxylases; Nutritional recovery
As we have for long time pointed out, pre-, peri- and postnatal undernourishment causes an activation of the brain serotonergic biosynthetic system that positively correlates with an elevation of plasma free fraction of L-Tryptophan (FFT) [1-4]. FFT crosses from plasma to the brain through the blood brain barrier (BBB) and is uptaken by brainstem serotonergic neurons to activate serotonin synthesis [3,5-8]. There is a FFT increase, and brain L-Trp also increases to levels higher than those of normal controls, showing that TPH activity is overactived, mostly in UNDO pups’ brain, with a significant change in its kinetics also being observed, as well as an increased affinity for its substrate and an increase in its phosphorylation capacity. These changes were considered to explain serotonin synthesis chronic elevation in the brain of animals that suffered intrauterine growth restriction (IUGR) secondary to undernourishment [9]. TPH is the serotonin biosynthetic pathway rate-limiting enzyme [10-12] and two isoforms have been described: TPH, apparently localized located only in peripheral tissues, and TPH2 [13-15], apparently present only in the central nervous system. In a recent study, our group reported the presence of TPH1 in the brainstem of the malnourished offspring, coexisting with the TPH2 isoform, with the former showing an ascendant developmental pattern during nursing and, interestingly, with significantly higher activity than that of TPH2 [16]. These findings suggest that 5-HT synthesis chronic elevation in the brain of IUGR pups might be due to a significant change of the TPH1 protein structure caused by early nutritional stress during fetal life, with a possible mediation of the Pet-1 regulatory molecular system [17]. However, when the undernourished offspring were subjected to a nutritional recovery regimen since the neonatal period, they showed a physical catch-up to normality, as well as a plasma biochemistry return to control values. But, despite this impressive recovery, TPH1 activity remained significantly elevated in their brainstem, together with an increase in the 5-HT neurotransmitter levels, which prevailed up to adulthood [18-20]. Bearing in mind all the above concepts, the present work was planned trying to obtain further information on the neurobiological mechanism implicated in the persistence of 5-HT increased synthesis in the brain of inutero nutritionally-stressed UNDO subjects, and find out whether TPH1 expression remains upregulated or returns to normal values after early nutritional recovery.
Female nulliparous Wistar rats were used (200 ± 10 g body weight). The rats were adapted for a period of two weeks to the following environmental conditions: temperature, 22 ± 2°C; 12 h light and dark periods, (07.00 to 19 h): minimal noise and handling; 50% to 60% relative humidity; rodent diet 5001 and water ad-libitum. At the end of this period, the animals were fed a rodent diet and were distributed as follows: An undernourished group that had food intake restricted to 50% of that consumed by the control group in 24 h (UNDO group). Controls were allowed a free ad-libitum diet (C group). After two weeks under this nutritional regimen, the females of both groups were mated to eutrophic males. During gestation, the animals were kept under the same general conditions. At birth, each group’s offspring were mixed and redistributed in litters of 8 members to mothers of their own group. Pups from the control group remained with their own mothers throughout the experimental period (group C), a subgroup of pups from UNDO group mothers were assigned to be fed by mothers of the C group for nutritional recovery (NR group). UNDO group mothers fed their own pups throughout the experimental period, which formed the permanently undernourished (UNDO) pup group. At ages 1, 15 and 21 days, the pups from all groups were weighed and anesthetized with sodium pentobarbital, 40 mg/kg body weight. A number of pups from each group were perfused through the intracardiac route with 0.9% saline solution and then with 4% p-formaldehyde. Then, they were sacrificed by neck dislocation, and the brainstem was dissected and placed in 4% p-formaldehyde, to be subjected to immunohistochemistry procedures in brainstem samples from the same groups. After dissection, tissues were immediately frozen in methyl butane and kept at -80°C, for subsequent Western blot assays for both TPH isoforms, and for L-Trp, 5-HT and TPH activity determinations. In addition, all offspring from all groups had their body weight and crown-rump length (CRL) determined. The criteria for undernourishment were a body weight and CRL deficit to a level significantly lower than 10% with regard to control body weight and CRL. In order to decrease circadian variations, experimental manipulations were always carried out at between 10 and 11.00 h. The Wistar rats were supplied by CINVESTAVIPN animal facilities. All experimental procedures were carried out following all international and national protocols for the care of laboratory animals.
Biochemical Assays
High-performance liquid chromatography (HPLC) was used for 5-HT and L-Trp determination, following the Peat and Gibb method [21], which has previously been described elsewhere. TPH specific activity was evaluated by 5-hydroxytryptophan (5-HTP) formation per mg/protein/h with HPLC fluorometric detection [22].
Immunohistochemistry for TPH
Brainstems were placed in a 4% p-formaldehyde solution for one week at 4°C and then embedded in paraffin to obtain 4 μM-thick sections. Sections were then placed on electrocharged slides. Subsequently, the sections were deparaffinized, and antigenic exposure was carried out using a citrate buffer (0.1 M, pH 6.0) (DECLERE, Cell Mark, Rocklin, CA, USA) in a microwave oven for three 2-min cycles. Endogenous peroxidase activity was immediately inhibited with 5% H2O2. After washing, the sections were incubated with TPH1-specific (Rabbit monoclonal antibody [EP1311Y] to Tryptophan hydroxylase 1 (TPH1), Catalog Number GTX61516, GeneTex Inc., Irvine, CA) and TPH 2-specific (Rabbit nonclonal antibody, [EPR19191] ab184505, abcam, Cambridge, United Kingdom) primary antibodies at a dilution of 1:500 in PBS solution (0.1 M, pH 7.4, Triton X-100) with 0.3% and 3% goat serum for 18 h at 4 ºC. On the following day, the sections were washed and incubated with the secondary antibody diluted in PBS and Triton X 100 (biotinylated anti-goat IgG antibody) for 30 min. Tissues were then incubated with avidin-biotin complex for 15 min at room temperature and washed. Immunoreactivity was determined with a commercial kit (3,3-diaminobenzidine and H2O2) according to Vector Laboratories’ protocol [23]. Photomicrographs were taken with a digital camera (Infinity 1-Lumenera). The number of TPH1 or 2-immunoreactive neurons in the DRN, for each age group and at each age, was determined in six 4 μM-thick sections, in an area of 83 μM2 with a 40X objective, using an Infinity 1-Lumenera camera equipped with a 10X objective, aided with an Olympus microscope.
TPH Immunotransference by Western blot
Brainstems were homogenized for 30 sec at 4°C in a solution of 50 mMTris-HCl, pH 7.4, plus protease inhibitors (Protease Inhibitor Cocktail, Sigma-Aldrich, St Louis MO, USA). The samples were then centrifuged at 29,000 g for 15 min at 4°C. Protein concentration was quantified by the Bradford method [24]. Then, 50 μg of protein were placed in each of the 1-mm-thick channels of a 12% SDS-polyacrylamide gel. The electrophoretic run was carried out at 100 V for 2 h. For protein electrotransference, the gel was mounted onto nitrocellulose sheets (BIO-RAD, USA) and the run was performed at 15 V, for 45 min, in a semi-dry chamber. Membranes with the transferred proteins were placed in a blocking solution (Millipore Chemiluminescent Blocker, Bedford, MA, USA) for 60 min. The membranes were incubated overnight with monoclonal primary antibody specific for TPH1 (Rabbit monoclonal IgG, Gene Tex Inc., Irvine, CA) or TPH2 (Rabbit polyclonal, Merck-Millipore) at a dilution of 1:1000 in the same blocking solution. The next day, the membranes were incubated with secondary anti-rabbit antibody (Goat Anti-Rabbit, IgG [H+L]-HRP conjugate, Bio-Rad, USA) at a dilution of 1:5000 in the same blocking solution. After washing the membranes with PBS, they were revealed by chemiluminescence with Luminata Forte Western HRP Substrate (Millipore, USA). Glyceraldehyde-3- phosphate dehydrogenase (GADPH) was used as internal control. The obtained bands were analyzed and quantified by densitometry.
To compare the serotonergic activity results, the number of TPH1 or TPH2-immunopositive neurons and serotonergic activity, (L-Trp, TPH activity and 5-HT concentration), as well as the relative densities of the Western blot bands observed in the DRN, were determined in all groups (ages 1, 15 and 21 days). For between-group differences, two-way ANOVA (nursing tine 1, 15 and 21 days and the mothers’ nutritional status, both controls, malnourished) and post-hoc analysis were conducted using Tukey’s Multiple Comparison test; P<0.05 was accepted as statistically significant.
Maternal nutritional restriction throughout gestation and nursing confirmed a previously reported deep undernourishment in their offspring in comparison with normally fed dams (p<0.001) (Figure 1). However, when undernourished pups were sufficiently fed by normal control dams, they showed an impressive physical recovery at the end of the nursing period (Figure 1). A significant increase in serotonergic system activity (L-Trp concentration, TPH activity and 5-HT level) in the brainstem was also confirmed with regard to the control group (P<0.05) (Figure 2). Interestingly, in the nutritionally-recovered pups (NR group), L-Trp level in brain tissue returned to normal values; however TPH activity remained significantly increased, as 5-HT concentration did (p<0.05) at the end of the nursing period (Figure 2).
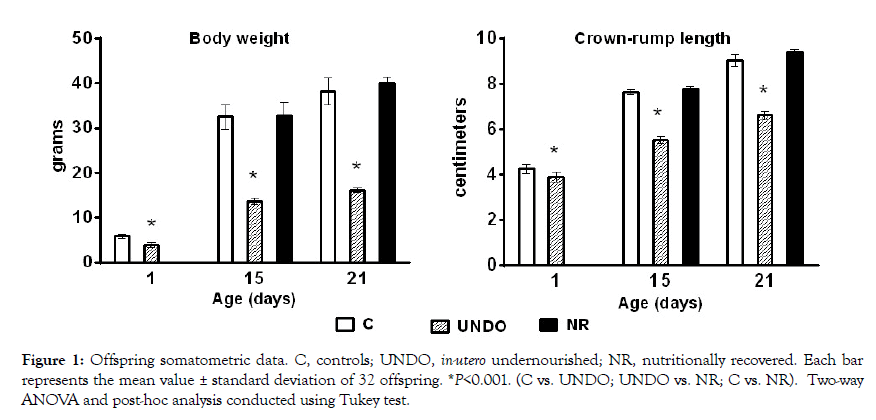
Figure 1: Offspring somatometric data. C, controls; UNDO, in-utero undernourished; NR, nutritionally recovered. Each bar represents the mean value ± standard deviation of 32 offspring. *P<0.001. (C vs. UNDO; UNDO vs. NR; C vs. NR). Two-way ANOVA and post-hoc analysis conducted using Tukey test.
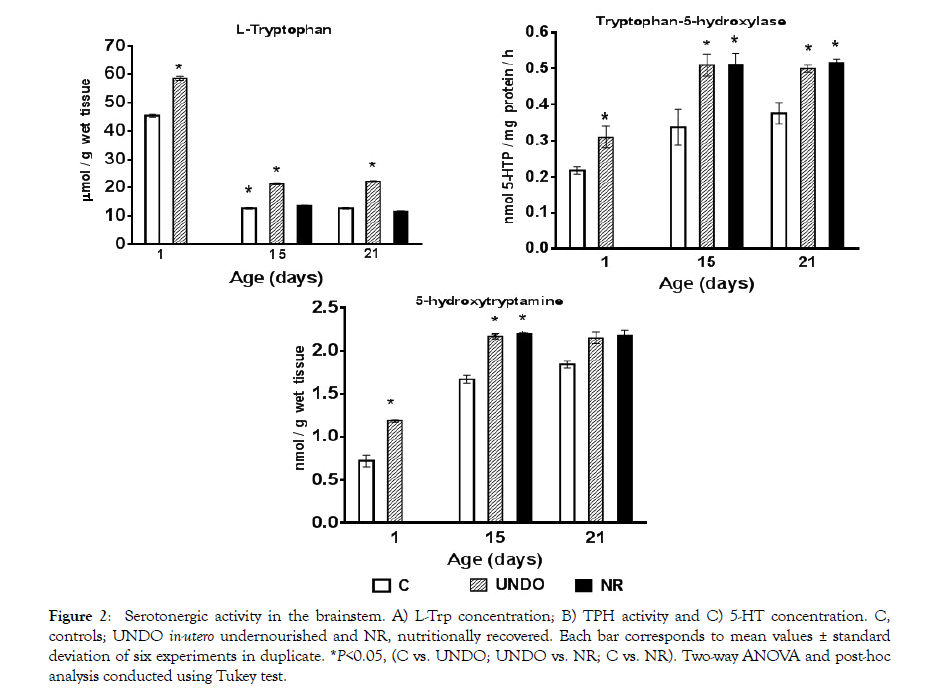
Figure 2: Serotonergic activity in the brainstem. A) L-Trp concentration; B) TPH activity and C) 5-HT concentration. C, controls; UNDO in-utero undernourished and NR, nutritionally recovered. Each bar corresponds to mean values ± standard deviation of six experiments in duplicate. *P<0.05, (C vs. UNDO; UNDO vs. NR; C vs. NR). Two-way ANOVA and post-hoc analysis conducted using Tukey test.
Figure 3A shows TPH isoforms expression in all experimental groups. A comparison of the expression of both TPH1 and TPH2 isoforms can be seen in Figure 3B, with TPH1 expression prevailing in the undernourished pups since birth (p<0.05). Interestingly, in the control group, TPH2 predominated throughout the nursing period (p<0.05), while the undernourished pups showed a decrease in TPH2 expression versus controls (p<0.05) (Figure 3B). In the nutritionally recovered group, TPH1 expression did not return to normal values and remained elevated, whereas the opposite was observed with TPH2, which returned to normal values with nutritional recovery until the end of the nursing period (Figure 3B).
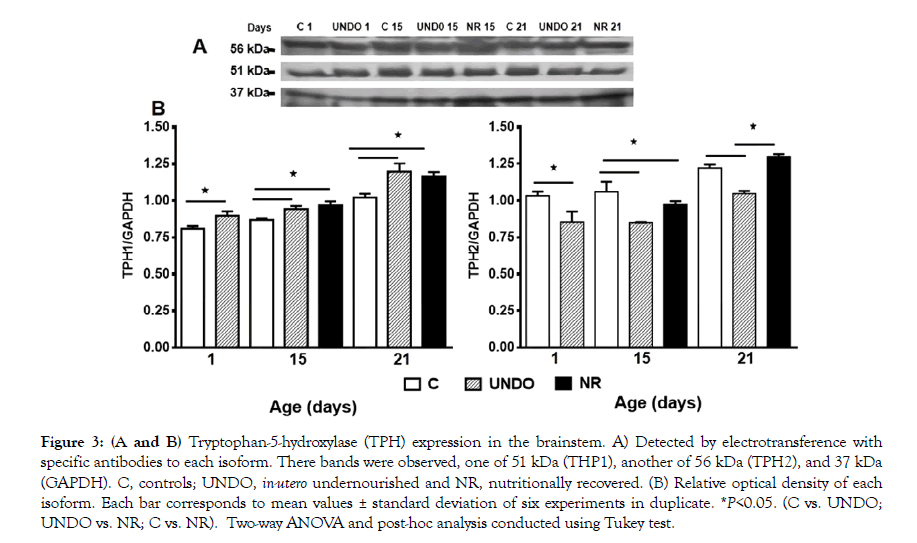
Figure 3: (A and B) Tryptophan-5-hydroxylase (TPH) expression in the brainstem. A) Detected by electrotransference with specific antibodies to each isoform. There bands were observed, one of 51 kDa (THP1), another of 56 kDa (TPH2), and 37 kDa (GAPDH). C, controls; UNDO, in-utero undernourished and NR, nutritionally recovered. (B) Relative optical density of each isoform. Each bar corresponds to mean values ± standard deviation of six experiments in duplicate. *P<0.05. (C vs. UNDO; UNDO vs. NR; C vs. NR). Two-way ANOVA and post-hoc analysis conducted using Tukey test.
Figures 4 and 5 show TPH1 or TPH2-immunoreactive neurons in the DRN from all groups, with a descending ontogenetic immunoreactivity pattern of both isoforms being observed in all groups. A population of 5-HT neurons was positive to TPH1 and another to TPH2 (Figures 4 and 5). Another remarkable result was that brainstem tissue from the undernourished group showed a significant decrease in the number of serotonergic neurons in comparison with the control group (P<0.05) (Figure 6). It is important underscoring that pups from the NR group showed a return to control values of decreased neuronal populations, which were, separately, immune-positive to both isoforms (Figure 6). Furthermore, in Figures 4 and 5, there seems to be some differences in neuronal morphological characteristics between the control group and the undernourished groups, including those that were nutritionally recovered.
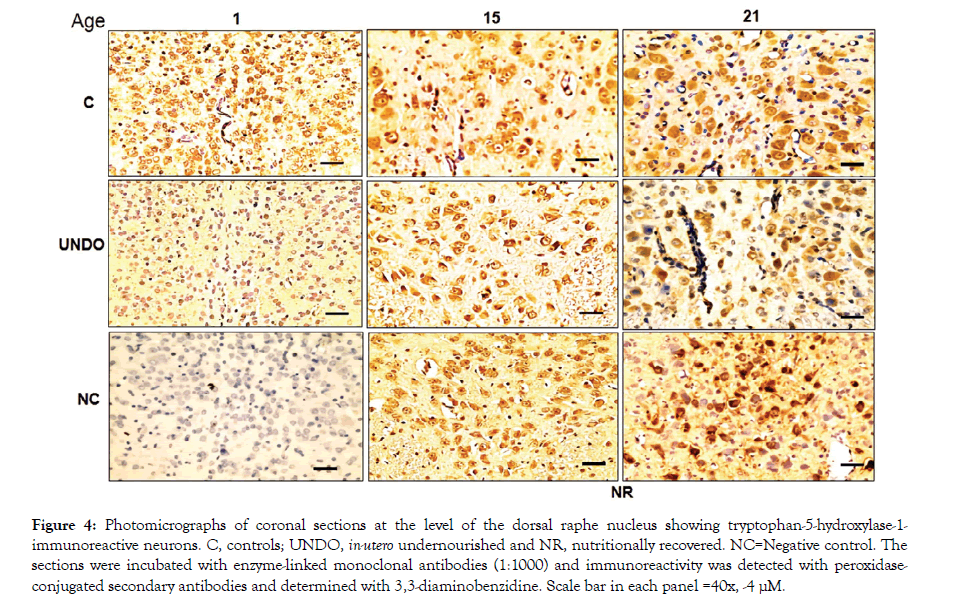
Figure 4: Photomicrographs of coronal sections at the level of the dorsal raphe nucleus showing tryptophan-5-hydroxylase-1-immunoreactive neurons. C, controls; UNDO, in-utero undernourished and NR, nutritionally recovered. NC=Negative control. The sections were incubated with enzyme-linked monoclonal antibodies (1:1000) and immunoreactivity was detected with peroxidaseconjugated secondary antibodies and determined with 3,3-diaminobenzidine. Scale bar in each panel =40x, -4 μM.
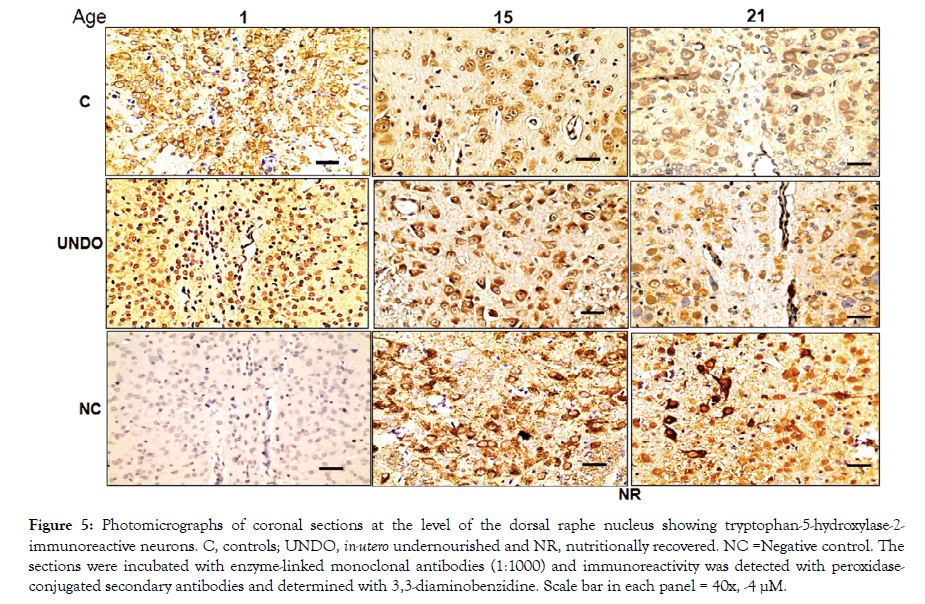
Figure 5: Photomicrographs of coronal sections at the level of the dorsal raphe nucleus showing tryptophan-5-hydroxylase-2-immunoreactive neurons. C, controls; UNDO, in-utero undernourished and NR, nutritionally recovered. NC =Negative control. The sections were incubated with enzyme-linked monoclonal antibodies (1:1000) and immunoreactivity was detected with peroxidaseconjugated secondary antibodies and determined with 3,3-diaminobenzidine. Scale bar in each panel = 40x, -4 μM.
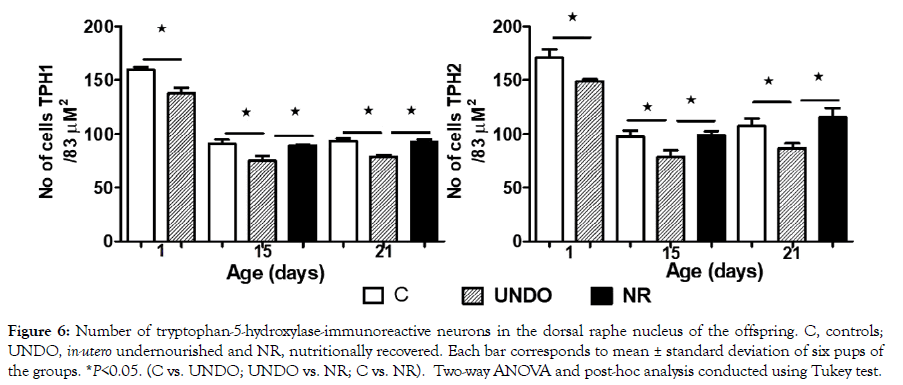
Figure 6: Number of tryptophan-5-hydroxylase-immunoreactive neurons in the dorsal raphe nucleus of the offspring. C, controls; UNDO, in-utero undernourished and NR, nutritionally recovered. Each bar corresponds to mean ± standard deviation of six pups of the groups. *P<0.05. (C vs. UNDO; UNDO vs. NR; C vs. NR). Two-way ANOVA and post-hoc analysis conducted using Tukey test.
The present work confirms and broadens reported results concerning undernourishment-related IUGR, which induces important neurobiochemical changes in brain serotonergic system during early development, further activated with a concomitant increase in 5-HT neurotransmitter levels, L-Trp concentrations and TPH1 activity in the brainstem, which in turn causes an activation of this important brain system in other brain areas, such as the cerebral cortex, and chronically increases its function during the perinatal and nursing periods, with this effect lasting up to adulthood in rats. Previously, we had also observed significant alterations in brain serotonin metabolism and morphological abnormal thalamocortical development [25,26]. With a noninvasive methodology, we were able to obtain interesting and significant information on biochemical and functional indicators of the sensory cortex also in human babies, which were markedly abnormal [2-4]. However, when early undernourished individuals were allowed to nutritionally recover, they showed a catch-up to normal physical values (including body weight and crown-rump length in rats) in comparison with controls, and body weight and length catch-up in human babies, as well as plasma FFT and L-Trp normalization in the brainstem in UNDO of rats. As in the present study, at the end of the nursing period, in spite of nutritional recovery, TPH activity remained significantly increased, accompanied by a corresponding increase in neurotransmitter concentration and function in the brain [18-20], which seems to be a relevant trait that persists up to adulthood in early undernourished rat pups, and to up to 3 months of age in human babies.
In a recent paper, we have also reported that, unexpectedly, both TPH isoforms (1 and 2) are expressed in serotonergic neurons since birth [16], showing an ascending developmental pattern during the nursing period in the rat brain, just as in the present study. It is important to mention that immunolabeling intensity was significantly higher for neurons labeled with the specific corresponding anti-TPH1 antibody. Interestingly, TPH2 showed a decrease in immunolabeling intensity at the end of the nursing period in comparison with the intensity shown by the TPH1 isoform [16].
Altogether, these findings support the contention that pre-, perior postnatal developmental undernourishment causes significant growth restriction and a possible change in TPH protein structure, supported by changes in its kinetics and phosphorylation capacity [9]. The presence of smaller number of serotonergic neurons expressing TPH1 agrees with a predominant expression of the enzyme protein in comparison to normal offspring. A significant decrease of TPH2 immunoreactive neurons and a lower concentration of the enzyme protein suggest that the early stressful conditions may induce an epigenetic influence, changing TPH expression to TPH1 predominance, whose mechanism is not clear. Together these results allow us to propose that prenatal stress may profoundly influence the biosynthesis of cerebral 5-HT [27-29], through mechanisms independent of genes encoding the enzyme protein, which in turn is caused by intense nutritional stress and to abnormal neurological changes caused by early undernourishment. Alternatively, these changes could be the product of modifications in Pet-1 (or FEV in humans) molecular assembly [17,30], which seems to be of key importance in the regulation of enzyme expression in the biosynthetic serotonergic pathway since very early in brain development [31].
Pet-1 knockout (KO) shows low TPH protein levels, and according to authors that have studied these molecular mechanisms, Pet-1 is also required for maintaining TPH enzymes in the developing brain. Thus, a significant alteration of Pet-1-related molecular mechanism can be advanced as a working hypothesis to obtain further information on the effects of early undernourishment on the activity of TPH enzymes in the developing brain. Another interesting possibility, to add further clarifying information to this long-lasting research project, is finding out whether the functional changes we have observed in tryptophan-5-hydroxylases and in plasma albumin protein and the metabolic consequences on the biosynthetic brain serotonin pathway, affect the brain sensory function in suckling human who suffered IUGR secondary to developmental undernutrition, and who were born to mothers with placental insufficiency, as well as to explore whether these changes are inheritable, a possibility that apparently would be more related to an epigenetic explanation.
Antonio Mondragon-Herrera Performed the Western blot and immunohistochemistry experiments. Analyzed the data and revised the writing of the article. Jorge Hernandez-Rodriguez. Helped in the execution of the experimental model in rat. He also revised the manuscript, Gabriel Manjarrez-Gutierrez. Designed the experimental model of intrauterine growth retardation and the different experiments. Also analyzed the results and wrote the manuscript.
The authors acknowledge the valuable assistance provided by Ismael Rodriguez, PhD, Alfonso Boyzo-Montes de Oca, PhD, José Carlos Guadarrama-Olmos and Marisol Bautista Torres.
This work was carried out thanks to the financial support granted by the Mexican Institute of Social Security (FIS/IMSS/PROT/ G15/1404).
The authors declare no conflict of interest.
The study was approved by the research and ethics committees of the Health Research Coordination, Mexican Institute of Social Security, Mexico City (R-2014-785-052). Material from other sources not reproduced.
Citation: Manjarrez-Gutiérrez G, Hernández-Rodríguez J, Mondragón-Herrera JA (2020) Nutritional Recovery and its Effect on Tryptophan-5-Hydroxylases Expression, Cell Number and on Changes Caused by Intrauterine Growth Restriction in the Developing Brain. J Nutr Food Sci. 10:774. doi: 10.35248/2155-9600.20.10.774.
Received: 27-Apr-2020 Accepted: 22-May-2020 Published: 29-May-2020 , DOI: 10.35248/2155-9600.20.10.1000774
Copyright: © 2020 Manjarrez-Gutiérrez G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.