Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2015) Volume 5, Issue 1
Microwave assisted extraction (MAE) was applied for inulin extraction from chicory and response surface methodology (RSM) was used to optimize the effects of processing parameters of extraction on the yield of inulin. A model equation was proposed to determine effects of solid: liquid ratio, microwave power (W), extraction temperature (?C) and extraction time (min). Conditions were optimised and tri-dimensional response surface plots were determined from the mathematical model. Maximum inulin extraction yield (63%) was obtained under the conditions of solid: liquid ratio (1:40), microwave power (400W), extraction time (30 min) and extraction temperature of 90?C. The yield of inulin content was higher in MAE compared to conventional extraction which resulted 51.20% of inulin. Microwave extraction thus can be used to extract inulin from chicory roots for its use in functional foods.
Keywords: Microwave-Assisted Extraction (MAE); Inulin extraction; Chicory; Response surface methodology
Inulin, a non-digestible carbohydrate, is a fructan that is not only found in many plants as a storage carbohydrate, but has also been part of man’s daily diet for several centuries. It consists of a long chain made up of 22-60 fructose molecules linked to each other by β (2→1) bonds, with a terminal glucose molecule. It is found in a variety of plant sources such as Jerusalem artichoke, chicory, dahlias, onion, garlic, banana, asparagus and leek. Amongst these vegetables, chicory roots are outlined for inulin production in industrial scale due to its higher content and stability in producing high Fructose/ Glucose ratio and because of its regular growing, even in moderate climates [1,2]. Inulin is mostly commercialized as a powder, which provides for convenient manipulation, transportation, storage and consumption. Degree of polymerization ranges from 2 to 60 depending on the source of inulin, time of harvest and the process of production [3].
Inulin can act as a sugar or fat substitute, with the advantage of exhibiting a very low caloric value. It used as an ingredient in foods with reduced or no sugar and fat, such as chocolates, ice creams and yogurt. Pure inulin is slightly sweet (10% sweetness in comparison with sugar), whereas high performance inulin (with a degree of polymerisation greater than ten) is tasteless. It acts in the human organs in a similar way as dietary fibres, contributing to the improvement of the gastrointestinal system conditions [4]. Many food and pharmaceutical industries have found applications for inulin in the production of functional foods, nutritional composites and medicines.
Various methods for extraction of inulin from chicory have been reported. In one method, the chicory roots were cleaned, sliced and extracted with hot water. The resulted thick juice was heated up to 95°C and then over a period of 30 hrs, cooled down to 4°C. The inulin precipitated, and was later filtered [5]. The existing methods are time consuming with less extraction efficiency. It is therefore necessary to find a suitable method for inulin extraction which is economical and produces high yield.
Microwave assisted extraction [MAE] is a process of using microwave energy to heat solvents during the extraction of the sample in order to partition analytes from the sample matrix into the solvent. The highly localised temperature and pressure can cause selective migration of target compounds from the material at a faster rate, thus providing enriched extracts compared to conventional extracts. It is a promising alternative sample preparation technique for a number of applications [6-9]. The ability to rapidly reach elevated temperatures and hold them for consistent times is the most important advantage of MAE [10]. Increase in extraction efficiency by microwave is generally attributed to its volumetric heating effect, which occurs due to dipole rotation of the solvent in the microwave field which causes the solvent temperature to rise, which then increases the solubility of the compounds of interest. The mechanism of the enhanced extraction by microwave assistance has been studied by observing cell destruction of plant material after MAE treatment, using scanning electron microscopy. Jian et al. [11] worked on the optimisation of microwave assisted extraction of polysaccharides from Cyclocaryapaliurus using response surface methodology.
When many factors and interactions affect desired response, response surface methodology (RSM) is an effective tool for optimizing the process and the main advantage of it is the reduced number of experimental trials needed to optimize the parameters [12,13]. RSM is a collection of statistical and mathematical techniques that has been successfully used for developing, improving and optimizing processes [14]. It has been successfully used to model and optimize the biochemical and biotechnological processes related to food systems [15-17], including extraction of phenolic compounds from berries, anthocyanins from black [18] and sunflower hull [19] and vitamin E from wheat [20].
Microwave assisted extraction of inulin from burdock roots was carried by XU Xin et al. [21] wherein the yield of inulin was 91.4% Wang et al. [22] could able to extract more than 92% inulin from Jerusalem artichoke with microwave treatment. The enhanced extraction of inulin using MAE treatment is due to cell rupture of the plant materials. It is in this context that conditions for microwaveassisted extraction of chicory roots were optimised. Response surface methodology was designed to optimise the essential parameters for the extraction of inulin, such as of solid: liquid ratio, microwave power, extraction temperature and extraction time, from chicory.
Materials
Matured chicory roots were obtained from Jupiter Food Products Private Limited, Etah, and Uttar Pradesh, India. The chicory roots were sliced and dried at 60°C in a cross flow drier for preservation. All chemicals used were obtained from Sigma-Aldrich (India) and were of analytical grade. The process of MAE was performed using a Microwave lab station (Model: STARTS configuration with control terminal 260, Milestone, Italy) at a frequency of 50Hz.s
Experimental design
RSM was applied to determine the working conditions of experiments to be performed using microwave lab station for the extraction of inulin from dried chicory. The central composite design (CCD) was employed to maximise the extraction yield. The effect of independent variables X1 (solid: liquid ratio), X2 (microwave power), X3 (extraction temperature) and X4 (extraction time) is checked for, at five coded levels in the extraction process (Table 1). The variables were coded according to the equation:
| Independent variables | Symbol | Levels | |||
|---|---|---|---|---|---|
| Un coded | Coded | -1 | 0 | 1 | |
| Solid: liquid ratio (w/v) | X1 | x1 | 20 | 30 | 40 |
| Microwave Power (W) | X2 | x2 | 400 | 600 | 800 |
| Temperature (°C) | X3 | x3 | 70 | 80 | 90 |
| Time (min) | X4 | x4 | 30 | 40 | 50 |
Table 1: Independent variable values of the process and their corresponding levels.
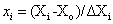 (1)
(1)
Where xi is the coded value of an independent variable, Xi is the real value of an independent variable, X0 is the real value of an independent variable at the centre point, and ΔXi is the step change value. The coded values were x1 (solid: liquid ratio), x2 (microwave power), x3 (extraction temperature) and x4 (extraction time) and they were given by the Eqns. (2)-(5):
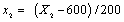 (2)
(2)
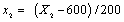 (3)
(3)
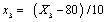 (4)
(4)
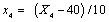 (5)
(5)
Yield inulin extraction was considered as a dependent response variable. The complete design consisted of 30 sets of experiments to verify the most significant factors affecting inulin yield. The experimental data was fitted to a second-order polynomial and regression coefficients obtained. The quadratic model for predicting the optimal points was expressed according to the following equation:
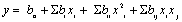 (6)
(6)
Where y is the response variable, bo, bi, bii and bij are the regression coefficients for intercept, linear, quadratic and interaction terms respectively. xi and xj are the coded levels of the independent variable. Data was analysed using Design Expert Software (Version 9.0, Statease Inc., and Silicon Valley, CA, USA).
Microwave assisted extraction
After the various combinations of processing conditions were obtained using RSM, as shown in Table 2, the experiments were performed. Different extractions were carried out by microwave heating of 1 g of dried chicory in different volumes of water. The slurry was filtered to yield a clear extract that was used for quantitative analysis. All samples were suitably diluted and filtered through a Hydrophilic PVDF (Polyvinylidene fluoride) membrane with 0.45 μm pore size and analysed for inulin content using high performance liquid chromatography.
| Run | Coded variable levels | Observed (Y1) | Predicted (Y0) | |||
|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x4 | |||
| 1 | -1 | -1 | -1 | -1 | 39.05 | 37.90 |
| 2 | 1 | -1 | -1 | -1 | 51.75 | 52.57 |
| 3 | -1 | 1 | -1 | -1 | 42.72 | 37.12 |
| 4 | 1 | 1 | -1 | -1 | 42.8 | 47.89 |
| 5 | -1 | -1 | 1 | -1 | 39.93 | 44.40 |
| 6 | 1 | -1 | 1 | -1 | 63.0 | 59.07 |
| 7 | -1 | 1 | 1 | -1 | 45.04 | 43.63 |
| 8 | 1 | 1 | 1 | -1 | 54.73 | 58.30 |
| 9 | -1 | -1 | -1 | 1 | 45.45 | 42.61 |
| 10 | 1 | -1 | -1 | 1 | 58.12 | 57.28 |
| 11 | -1 | 1 | -1 | 1 | 40.5 | 41.84 |
| 12 | 1 | 1 | -1 | 1 | 54.03 | 56.51 |
| 13 | -1 | -1 | 1 | 1 | 35.3 | 34.38 |
| 14 | 1 | -1 | 1 | 1 | 40.11 | 48.27 |
| 15 | -1 | 1 | 1 | 1 | 39.6 | 33.61 |
| 16 | 1 | 1 | 1 | 1 | 50.53 | 48.28 |
| 17 | -2 | 0 | 0 | 0 | 17.01 | 23.76 |
| 18 | 2 | 0 | 0 | 0 | 61.28 | 53.09 |
| 19 | 0 | -2 | 0 | 0 | 60.5 | 58.92 |
| 20 | 0 | 2 | 0 | 0 | 57.22 | 57.36 |
| 21 | 0 | 0 | -2 | 0 | 44.62 | 45.26 |
| 22 | 0 | 0 | 2 | 0 | 49.63 | 44.40 |
| 23 | 0 | 0 | 0 | -2 | 47.33 | 47.91 |
| 24 | 0 | 0 | 0 | 2 | 39.11 | 42.61 |
| 25 | 0 | 0 | 0 | 0 | 44.62 | 45.26 |
| 26 | 0 | 0 | 0 | 0 | 44.61 | 45.26 |
| 27 | 0 | 0 | 0 | 0 | 44.63 | 45.26 |
| 28 | 0 | 0 | 0 | 0 | 44.62 | 45.26 |
| 29 | 0 | 0 | 0 | 0 | 44.63 | 45.26 |
| 30 | 0 | 0 | 0 | 0 | 44.64 | 45.26 |
Table 2: Central Composite Design with the observed responses and predicted values for inulin yield (%).
Conventional extraction
Dried chicory was extracted with water at boiling temperature for 30 min. The slurry was filtered to get a clear extract that was used for quantitative analysis [5].
HPLC analysis
Sample analysis was performed using a Waters HPLC 2695 equipped with a quaternary pump and Empower software. The detector was ELSD 2424 (Waters model). An Aminex HPX-87C column (300 × 7.8 mm i.d.) was used at a column temperature of 25°C. The mobile phase was degassed triple distilled water at a flow rate of 0.5 ml/min. The sample injection volume was 20 μl. ELSD conditions were as follows: nebulizer temperature 12°C and drift tube temperature 50°C. For the inulin quantitation, a commercial standard (Sigma 12255-10G) was used.
Inulin, a nondigestable carbohydrate, is a fructan that is not only found in many plants as a storage carbohydrate, but has also been part of man’s daily diet for several centuries. Inulin has a remarkable capacity to replace fat. When thoroughly mixed with water or another aqueous liquid, it forms a particle gel network resulting in a white creamy structure with a short spreadable texture, which can easily be incorporated into foods to replace fat by up to 100% [23]. Inulin and oligofructose are functional food ingredients, which offer a unique combination of nutritional properties and important technological benefits. The food industry has found major applications for the inulin derived or associated products, mostly because it combines easily with other food ingredients and contributes towards the improvement of organoleptic properties. Recently, it has been shown that modified inulin can be exploited as a chelating agent, a detergent co-builder and pharmaceutically as a carrier for drug delivery.
Statistical analysis
In this study, Design-Expert software (Version 7.0.0, Stat-Ease Inc., Minneapolis, USA), was used with BBD (Box-Behnken Design)-RSM based design approach. On the basis of the BBD, the process parameters such as solid: liquid ratio, microwave power, extraction temperature and extraction time was optimised for maximum yield of inulin.
Fitting the model
A regression analysis (Table 3) was carried out to fit the response variable with the experimental data. Some insignificant terms like 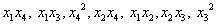 were removed. Terms like
were removed. Terms like 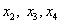 were kept to preserve model hierarchy. The significance of each coefficient was determined using the F-test (ANNOVA) p-value in Table 3.
were kept to preserve model hierarchy. The significance of each coefficient was determined using the F-test (ANNOVA) p-value in Table 3.
| Source | SS | Df | MS | F Value | p-valuea |
|---|---|---|---|---|---|
| x1 | 1290.96 | 1 | 1290.96 | 55.71 | <0.0001+ |
| x2 | 3.62 | 1 | 3.62 | 0.16 | 0.6982+ |
| x3 | 4.44 | 1 | 4.44 | 0.19 | 0.6679+ |
| x4 | 42.19 | 1 | 42.19 | 1.82 | 0.1972+ |
| x1x2 | 22.61 | 1 | 22.61 | 0.98 | 0.3389 |
| x1x3 | 5.66 | 1 | 5.66 | 0.24 | 0.6282 |
| x1x4 | 0.81 | 1 | 0.81 | 0.035 | 0.8542 |
| x2x3 | 41.86 | 1 | 41.86 | 1.81 | 0.1989 |
| x2x4 | 12.46 | 1 | 12.46 | 0.54 | 0.4747 |
| x3x4 | 217.12 | 1 | 217.12 | 9.37 | 0.0079+ |
| x1^2 | 72.71 | 1 | 72.71 | 3.14 | 0.0968+ |
| x2^2 | 298.81 | 1 | 298.81 | 12.90 | 0.0027+ |
| x3^2 | 42.99 | 1 | 42.99 | 1.86 | 0.1933 |
| x4^2 | 10.19 | 1 | 10.19 | 0.44 | 0.5174 |
Table 3: Analysis of variation for Response Surface Quadratic model for inulin yield % (before model reduction).
The predicted model can be described by the following equation in terms of coded values:
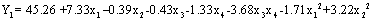 (7)
(7)
The significance of the second-order polynomial model equation was checked by an F-test (ANOVA) and p-value. The corresponding variables would be more significant if the absolute F-value becomes greater and the p-value becomes smaller [14].
The result suggest that the change of solid: liquid ratio (p<0.0001), interaction between extraction temperature and extraction time (p=0.0079) and microwave power had considerable effects on the yield of inulin when MAE was used to extract inulin from chicory. It can be seen that the variables with the largest effect were the linear terms of solid: liquid ratio (x1), interaction term of extraction temperature and extraction time (x3x4) and quadratic term of microwave power (x42).
The fit value, termed R2 (determinant coefficient) of the polynomial model was calculated to be 0.8009, indicating that 80.09% of the variability in the response could be explained by the second-order polynomial prediction equation. The Model F-value of 12.64 implies the model is significant. There is only a 0.01% chance that an F-value this large could occur due to noise. The experimental values were reasonably close to the predicted values confirming the validity and adequacy of the predicted models. Thus the ANOVA results show that this model is appropriate. In order to express the effects of processing parameters, the following equation is given in terms of actual factors:
The equation in terms of actual factors is:
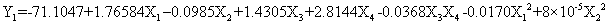 (8)
(8)
Response surface analysis
The relationship between independent and dependent variables is shown in tri-dimensional representation of the response surfaces and two dimensional contour plots generated by the model for inulin (Figures 1 and 2). In tri-dimensional surface plots, the effects of two variables on the response were depicted while the two other variables were kept constant.
Figure 1 shows the response surface plots and contour plots of the effects of amount of solvent (ml) and microwave power on the inulin yield. The amount of solvent displays a significant linear increase on the yield of inulin. This indicates that higher volume of solvent (lower solid: liquid ratio) led to a higher response. This is due to availability of more liquid which increases the driving force of inulin out of the root [24]. Also, large concentration difference favours mass transfer. However, this observation was different from MAE studies performed by Eskilsson & Bjorlund [25] according to which, higher amount of solvent gives lower recovery.
The microwave power plays a quadratic effect on the response. Microwave absorption results in significant internal heating thus creating significantly higher internal pressures which enhance inulin extraction from the chicory. The cell walls can swell and burst because of internal heating and thus further accelerate the intracellular product release into the solvent. In the present study, extraction of inulin was maximum when microwave power of 400W. The same result was observed during the extraction of inulin from burdock roots using microwave power at the wattage of 400 [26] and also the microwave extraction of polysaccharides from Rananculus ternatus [27].
The response surface and contour plots in Figure 2 depicts the interaction effects of the two dependant variables such as extraction temperature and time. Both time and temperature demonstrate a constant effect on the response. These variables individually do not have much effect on inulin extraction, but their interaction with each other plays an important role. It has been cited that increase in polysaccharide yield is due to the strong effect of extraction time-temperature on the mass transfer rate of the water soluble polysaccharides in the cell wall [28-29]. Extraction temperature of 90°C was optimised because maximum inulin yield was obtained at this temperature. The same temperature was used by Wang Qi-wei et al. [30] to extract inulin from Jerusalem artichoke using microwave treatment.
It can be observed that inulin yield decreased slightly as the time increased from 30 min to 50 min. highest response is obtained at 1:40 solid: liquid ratio when the time is 30 min. According to the reports of [31,32], inulin is a polydisperse carbohydrate, and a part of the inulin fractions may be easily degradable. The internal heating due to microwave may cause partial hyperthermia, so the easily degradable inulin fractions may be degraded to mono-saccharides, thus decreasing the inulin recovery.
The plots show that inulin extraction reached its maximum at a combination of 1:40 solid: liquid ratio, extraction power of 400W, temperature 90°C and extraction time of 30 min. A maximum inulin yield of 63% occurs at these points.
Comparative analysis of MAE and conventional extracts
The comparative analysis of extraction efficiency of inulin yield between conventional heat reflux method and MAE under optimum conditions is shown in Table 4. It is clearly evident from the comparative data that MAE shows significantly higher and better extraction yield than traditional method under optimum conditions. MAE using aqueous phase leads to rapid energy transfer between polar molecules. Water molecules in the cells, because of their higher dipole moment, absorbs microwave energy strongly, leading to efficient heating of the sample. In the conventional heat reflux extraction process, the solvent transfers into the matrix and extracts the compounds by solubilisation. But in MAE, the selective interaction between the internal free water molecules and microwave leads to rapid increasing of temperature and causes expansion with subsequent rupture of the cell walls. Such a system undergoes a dramatic expansion and subsequent rupture of the cell walls, allowing the release of the compounds into the solvent [22]. Therefore the rupture of cell walls and migration of compounds out of the cells into solvent in MAE was both easier than that in heat reflux extraction.
| Exp. Set | Method of Extraction | Parameters | Inulin Yield % |
|---|---|---|---|
| I | Conventional heat reflux method of extraction | Time (30 min) Temperature (at boiling) Solid: Liquid ratio (1:40) | 51.20 ± 0.14a |
| II | Microwave–assisted extraction (MAE) | Time (30 min) Temperature (90°C) Power (400W) Solid: Liquid ratio (1:40) |
63.00 ± 0.04b |
Table 4: Comparative analysis of MAE and conventional extracts. Values are Means ± SD of triplicates; values having different superscripts are significantly (p<0.05) different.
Microwave-assisted extraction has been considered as a potential alternative to traditional solid-liquid extraction for the isolation of inulin yield from chicory roots. The present study has focussed on MAE of inulin from chicory roots. The conditions were optimised using RSM and the optimum values for solid: liquid ratio, microwave power, temperature and time were found to be 1:40, 400W, 90°C and 30 min. Under the optimum conditions of power, temperature and time, maximum inulin could be extracted and these concentrations were higher than those after the heat reflux extraction at boiling for 30 min. The solid: liquid ratio, interaction between temperature and time, and the quadratic of power had significant effects on the response value. The optimum extraction conditions for inulin showed that using MAE will increase the extraction of inulin from chicory roots. This may be attributed to the better absorption of microwave energy, which increases temperature inside plant cells, resulting in the breaking of plant cells and releasing compounds into the surrounding solvent. Thus as compared to conventional techniques, MAE provide higher efficiency. Hence, this method can be used selectively to prepare inulin rich conserves from chicory roots for use in functional foods.
The authors are grateful to the Director, Central Food Technological Research Institute, Mysore; Head, Human Resource Development and Dean, School of Biosciences and Technology, VIT University, Vellore for providing facilities and encouragement.