Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Review Article - (2017) Volume 7, Issue 3
Pulsed Electric Field (PEF) treatment is a non-thermal food processing technology that mostly applied to low viscosity high acidity food samples. Due to its non-thermal nature, it is preferred over thermal processing technologies since it has a superiority to preserve physical, chemical, biochemical and sensory properties of food with extended shelf life. Studies focused on preservation of bioactive and health-related compounds usually involve effect of PEF on water soluble vitamins, total antioxidant capacity, total phenolic compounds, phenolic substances, organic acids, and anthocyanins and are limited on how bioaccessibility and bioavailability of these compounds are changed by PEF processing. Thus, effects of PEF processing on bioaccessibility and bioavailability of food components with both in vivo and in vitro studies emphasizing on future needs are emphasized in this review.
Keywords: Pulsed Electric Field (PEF); Bioavailability; Bioactive compounds; Bioactivity
PEF: Pulsed Electric Fields; HPP: High Pressure Processing; TT: Thermal Treatment; HAA: Hydrophilic Antioxidant Activity; BC: Bioactive Compound; WB: Water-Fruit Juice Beverage; MB: Milk-Fruit Juice Beverage; SB: Soymilk-Fruit Juice Beverage; TMP: Transmembrane Potential
Pulsed Electric Field (PEF) treatment is defined as the application of short burst of high intensity electric field pulses in the range of 20-80 kV/cm for very short treatment time of micro to milliseconds to pasteurize foods [1-4]. PEF processing usually applied at ambient or little under or above ambient temperature and in addition to short processing time, heat generation during PEF process is minimized, and process remains non-thermal. After it is first practiced in 1930s, now it is one of the most studied non-thermal emerging technologies to process low viscosity high acidity food products especially fruit and vegetable juices, milk with low amount of fat, soups, and sauces [2,5-8].
PEF processing as a function of electric field strength, electrical energy and treatment time does not cause detrimental changes on physical, biochemical and sensory properties of food samples as well as bioactive compounds. Moreover, PEF processing provides inactivation of spoilage and foodborne pathogens as well as enzymes that cause microbial spoilage and downgrading quality, respectively [2,9-12]. Studies with microbial reduction involve inactivation of Escherichia coli, Escherichia coli O157:H7, Salmonella sp., Listeria monocytogenes, Listeria innocua, Bacillus cereus, Pseudomonas fluorescence and Saccharomyces cerevisiae, etc. [13-19], whereas enzyme inactivation studies include inhibition of Pectin Methyl Esterase (PME), lipoxygenase, polygalacturonase, Peroxidase (POD), Polyphenoloxidase (PPO), and ß-glucosidase [20-31].
Killing of vegetative bacteria and yeasts by PEF processing is probably not due to the products of electrolysis or temperature increase alone, but rather by the applied electrical field strength and the processing time [32-34]. There are a few theories on the mechanisms involved in the disruption of the cell membrane when subjected to electric fields. Two hypotheses, electrical breakdown and osmotic disproportion, are widely approved and are supported on the same principles [34]. The theory of electrical breakdown considers the cell membrane as a condenser loaded with a dielectric medium [34,35]. Accumulation of free charges at the internal and outer surface of the cell membrane forms a Transmembrane Potential (TMP) of approximately 10 mV [34,36]. When an external electrical field is applied; ions inside and outside of the cell move along with the electric field until they are restrained and accumulated at the membrane causing a rise in the TMP. The ions of opposite charge (+ and –) on each side of the membrane are pulled to each other, squeeze the membrane and cause a decrease in its thickness. Further application of electric fields causes more stress on cell membrane and reduction in its thickness ends up with pore formation when applied electric field strength is above the critical electric field potential of the cell membrane. If the application of electric field continues, the pores become irreversible and cells cannot reseal themselves leaking of intracellular materials, and thus, cell death occurs [34,37]. The principle of osmotic irrationality, on the other hand, defines the imbalance of cell membrane components through the formation of hydrophilic pores in the membrane and the opening of the protein channels. Applied electric field causes structural changes in the conformation of phospholipids, ending up the rearrangement of the membrane structure and constitution of hydrophilic pores [34,38].
Although inactivation mechanism of enzymes by PEF is not clear, the scientific literature proposes that electrochemical effect of PEF provides changes in the structure and conformation of enzymes that cause inactivation [34,39]. Protein structures are preserved by a delicate balance of covalent peptide bonds and non-covalent interactions of hydrogen bonds as well as hydrophobic, electrostatic, and Van der Waals interactions. The electric field application may influence the territorial electrostatic forces in proteins and break electrostatic interactions of peptide chains giving rise to conformational changes [23,24,40-48].
Studies with PEF pasteurization of foods mostly focused on microbial inactivation, changes in physical, chemical and sensory properties in addition to shelf life extension. With the increasing demand on nutritional quality of foods, fate of bioactive compounds after processing are being studied not only by heat but also PEF processing. Effect of heat processing on bioactive compounds as well as bioaccessibility and bioavailability are well established, but not enough information is available about the effect of PEF on bioaccessibility and bioavailability of foods. Thus, more attention needs to be given to current studies exploring effect of PEF on bioaccessibility and bioavailability of food components as well as future needs and trends for the determination of the impact of PEF on bioactive food compounds.
Bioavailability is described as the rate of nutrients, bioactive compounds or phytochemicals that are assimilated, absorbed and metabolized through normal pathways [49,50]. Bioavailability term has many descriptions regarding the research field. In point of the nutritional view, bioavailability refers to ingested bioactive composition that is available for utilizing in physiologic acts or to be stock [51,52]. According to Benito and Miller [53] and Fernández- García et al. [52]; bioavailability is defined as a quantity of a precise nutrient in a certain food that the organism can actually utilize. Bioavailability is an important term in terms of the nutritional impact of the food [52,54]. The organism can use exclusively determined amounts of all nutrients and bioactive ingredients in food. The bioavailability term involves availability for absorption, metabolism, bioactivity and tissue circulation. On the other hand, there are some practical and ethical troubles to evaluate in point of the food components’ bioactivity and circulation on particular organ sites. Therefore, the fraction of an oral dose of a basic compound reaching organism circulation can be described as bioavailability. Bioavailability and bioaccessibility terms are nested but the dual terms can be used indefinitely. Moreover, it must be known that bioavailability also subcategorizes bioaccessibility [52,55].
According to Fernández-García et al. [52] and Benito and Miller [53] bioaccessibility can be described as the part of a compound that is left from its matrix in the gastrointestinal region and therefore, becomes usable for digest absorption. Bioaccessibility is related with digestive transformation of food that can be absorbed by the organism. Bioaccessibility connotes both the absorption and dissimilation into the cells of the gastrointestinal epithelium and calculated as in Equation 1.
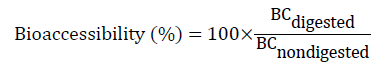 (1)
(1)
Where BCdigested refers to the bioactive compound concentration in the digested food and BCnondigested refers to bioactive compound concentration in non-digested food [56].
A term bioactivity first defined at the ESB consensus conference of 1987 as the “one which has been designed to induce specific biological activity” [57-82]. After that, different approaches have been made to define bioactive materials as bone bonding materials and it was more specifically stated as “…the essential requirement for a material to bond to living bone is the formation of bone-like apatite on its surface when implanted in the living body”, and that “…this in vivo apatite formation can be reproduced in a Simulated Body Fluid (SBF) with ion concentrations nearly equal to those of human blood plasma” [81]. The latest definition for bioactivity “a bioactive material is a material on which bone-like hydroxyapatite will form selectively after it is immersed in a serum-like solution” [81,83].
How the bioactive compounds are carried and transported to the target tissue, interactions with biomolecules, undergoing metabolism or transformation, biomarker generation, and caused physiologic responses are the answer of bioactivity term. Bioactivity is relevant with the interaction on biomolecules. At the same time bioactivity includes bioactive compounds metabolism and transportation, physiological reactions in tissues (Figure 1) [52].
Figure 1: Bioavailability definition including bioaccessibility and bioactivity [52].
Availability rate of bioactive compounds is more important than amount of bioactive compounds existed in food products. Bioavailability of a certain compound is affected by different factors such as processing technology, storage conditions or food matrix and most importantly by heat processing as degradation, decomposition or structural changes occurred by processing that can change the bioavailability of a certain compound. Thus effects of non-thermal technologies such as PEF and High Pressure Processing (HPP) are on search as it is predicted that these technologies can positively affect or develop the bioavailability of bioactive compounds [57,58].
Effects of PEF on bioactive properties and bioavailability
Healthy and nutritious products always are desired and preferred by consumers. Improving new preservation techniques are also important to maintain food freshness and guarantee stability of bioactive compounds with minimum physical and chemical processes [41,59,60-64]. Moreover, some researches demonstrated that bioactive properties of fresh fruit juices are maintained better after PEF application [41,59,60-64]. It has been reported that PEF causes less modification in the content of vitamins when compared to conventional heating processing treatments. Studies mostly conducted with determination of the fate of the ascorbic acid (Vitamin C) due to its sensitive nature; and it was revealed that PEF usually provided better preservation of vitamin C as it is compared to heat processing [58]. Similar results were reported for vitamin A, other water soluble vitamins, polyphenols, isoprenoid compounds, fatty acids and amino acids [58]. However, researches are insufficient in terms of the effect of PEF application on health benefits of fruits and vegetables.
Impact of PEF on bioavailability of vitamin C in a healthy human population consumed PEF treated fruit and vegetable products were also investigated [57,58,62,65]. Orange juice and a vegetable soup “gazpacho” were the treated by PEF (35 kV/cm, bipolar 4 μs pulse width at 800 Hz frequency, for 750 μs treatment time) and their bioavailability were tested on healthy human subjects. It was reported that vitamin C bioavailability in PEF-treated orange juice and gazpacho remained significantly higher after 14 days of storage at 4°C, compared to untreated samples. It is demonstrated that PEF provides an increase in the extraction of vitamin C from fruits and vegetables and, thereby, increase their health benefits [62]. Moreover, an improved in vitro bioaccessibility of vitamin C and phenolic compounds as well as the antioxidant capacity of PEF-treated (35 kV/cm, 1800 μs) fruit juice-based beverages compared to thermal processing (90°C, 60 s) was also reported [56].
Assessment of the bioavailability of vitamin C from PEF–treated orange juice in comparison with freshly squeezed and its impact on 8- epi PGF2α concentrations (biomarker of lipid peroxidation) in a healthy human was conducted with six subjects consumed 500 mL/day of PEF–treated orange juice and six subjects consumed 500 mL/day of freshly squeezed orange juice for 14 days, corresponding to an intake of about 185 mg/day of ascorbic acid. On the first day of the study, subjects drank the juice in one dose, and on days 2-14 they consumed 250 mL in the morning and 250 mL in the afternoon. Blood was collected every hour for 6 h on the first day and again on days 7 and 14. A maximum increase in plasma vitamin C occurred 4 h post dose in the dose-response study. Vitamin C remained significantly higher on days 7 and 14 in both orange juice groups. Plasma 8-epi PGF2α concentrations were lower at the end of the study in both groups and plasma levels of vitamin C and 8-epi PGF2α were inversely correlated. It was concluded that PEF–preservation of orange juice retains the vitamin C bioavailability and antioxidant properties of fresh juice with a longer shelf-life [65].
The study regarding the vitamin C bioavailability was carried out into two steps, a dose-response test and a multiple-dose response. For the dose-response measurement, the subjects consumed were asked to consume a 500 mL of juice or gazpacho after a minimum of 12 h of fasting, and blood samples were drawn before and every 60 min for 6 h. For the multiple dose response, the subjects were instructed to drink the juice or gazpacho at home, in two doses, 250 mL in the morning and 250 mL in the afternoon, for 2 consecutive weeks. Blood samples were taken again during the intervention on 7th and 14th days of the study. The maximum increase in plasma vitamin C occurred 3-4 h post-dose in both the HPP and PEF-treated groups for both products. Compared with the baseline, the vitamin C concentration was significantly higher on day 7 and 14 of the intervention in both men and women. It is also stated that, consuming PEF-treated orange juice or gazpacho of daily caused an increase in plasma vitamin C, improved the vitamin C bioavailability and provided a longer shelf-life of fresh products in addition to decline in oxidative stress in healthy humans [66].
Effect of food matrix on bioaccessibility
Effect of the food matrix (water-, milk- and soymilk- fruit juice beverages) and processing (PEF, HPP and thermal treatment, TT) on the in vitro bioaccessibility of vitamin C and phenolic compounds, as well as on the hydrophilic antioxidant activity of beverages based on a blend of fruit juices (orange, pineapple, kiwi and mango) was investigated. It was reported that PEF and HPP improved or did not change the bioaccessibility of vitamin C and certain phenolic compounds in comparison with untreated beverages. In contrast, TT diminished the bioaccessibility of most of the compounds that were measured. Soymilk-fruit juice beverages (SB) showed the greatest vitamin C bioaccessibility whereas water-fruit juice beverages (WB) favored the bioaccessibility of phenolic compounds and HAA. Bioaccessibility of these hydrophilic constituents reduced in milk-fruit juice beverages (MB). Bioaccessibility of vitamin C and phenolic compounds in fruit juice-based beverages was modulated by both food matrix and processing. Moreover, PEF allowed obtaining beverages with improved nutritional and functional quality [56]. In addition to processing technologies, the food matrix had a significant influence on the bioaccessibility of vitamin C. Soy-based products showed the highest bioaccessibility of vitamin C followed by WB and MB. It was also reported that the stability of vitamin C is influenced by several factors, such as oxygen availability, temperature, light, pH, metal catalyst, the presence of other antioxidants and reducing agents, as well as possible presence of ascorbic acid oxidase [67].
Fruits and vegetables contain most of phytochemicals as free or soluble forms. Total phenolic compounds exist of 24% on average in food matrix as bound phenolic compounds [68-70]. Phenolic compounds as bound forms can be released and absorbed by the organism. For instance, after coffee drinking, the level of caffeic acid in plasma rises because of the release and absorption of caffeic acid from bound complexes [70,71].
Absorption pathways of bound phenolic compounds are somewhat different in human as to intestinal system that some kind of microorganism and enzymes manage the absorption and releasing reactions. After the release of bound phenolic compounds in the gastrointestinal lumen, they tend to be absorbable and metabolized; and bound phenolic compounds become more useful for health [70]. Consumption of free or bound phenolic compounds provides beneficial health effect. Intake of bound phenolic compounds protects the organism against colon cancer. In addition, the intake of free or soluble conjugated forms of bound phenolic compounds is more effective in terms of the quick absorption in stomach and distribution through the body. In point of ensuring health benefits such as prevention the oxidation of LDL cholesterol and liposomes, both intake and release of free and soluble conjugated forms of bound phenolic compounds are important [70,72]. There are many ways to enhance the releasing of bound phenolic compounds and PEF is an effective way to assist hydrolysis reactions since PEF application of plant tissues enhances the porosity of cells [70,72,73]. For example, PEF application on red cabbage provided a substantial increase in the content of anthocyanins in extracts [70,73,74].
Both the amount and presence of vitamin C are important as it is necessary for the biosynthesis of collagen and hormones by the organisms in addition to alleviation of some kind of diseases such as cancer and cardiovascular diseases [56,75]. Moreover, dietary intake rich in phenolic compounds reduces neurodegenerative diseases and cancer [56,75,76] and heat processing changes the food structure, content or bioavailability of bioactive compounds. Eventually, these structural changes affect the bioactive compounds during digestion in the intestinal lumen regarding release, transformation and absorption of some nutriments [56,75,77].
Bioaccessible fraction is more significant than the amount of these ingredients to determine nutritional value of beverages. In addition, impact of food matrix on the bioaccessibility of bioactive compounds must be defined fully in beverages because of their complex structures as well as interaction of bioactive compounds and other constituents’ with each other [56,78,79]. Therefore, composition of food matrix and determination of the appropriate food processing method has a vital importance to understand bioaccessibility of bioactive compounds and interactions of food ingredients. Both in vitro and in vivo studies are more valuable and useful to estimate stability and bioaccessibility of bioactive compounds from food [56,80].
Whereas the vitamin C bioaccessibility is the highest in soymilkfruit juice beverage, the bioaccessibility of phenolic compounds and HAA are better in water-fruit juice beverages (mix of 75% of the blended orange, kiwi, pineapple and mango fruit juice with water). Conversely, bioaccessibility of vitamin C and hydrophilic constituents of phenolic compounds are not much better in milk-fruit juice beverage [56]. That’s why food matrix and method of the processing is important on the bioaccessibility of bioactive ingredients. As a result, all conditions as food matrix, application and bioactive compounds should be determined cleverly because bioavailability and bioaccessibility may reduce of bioactive compounds [56].
Recent trends from the food industry are to attract consumer’s attention through functional foods and beverages with high nutritious content with healthy concept as well as being easy to prepare and consume. Current food processing technologies provide safer food with longer shelf-life but often fail bioaccessibility and bioavailability of food components. Thus, more and more attention has been given to non-thermal technologies. It was shown by the limited number of in vivo and in vitro studies that PEF processed juices have better nutritional properties as well as superior in terms of bioaccessibility and bioavailability. As known, while most of the studies concentrate on the impact of PEF related to bioactive compounds properties and changes in quantity of foods, researches are fairly limited pointing the PEF effect on bioaccessibility and bioavailability in gastrointestinal system. Therefore, future studies need to focus on PEF processing of different fruit and vegetable juices with their impact on human health in addition to their bioaccessibility and bioavailability.