Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2016) Volume 6, Issue 5
Many of the health benefits associated with Aloe vera have been attributed to the polysaccharide contained in the gel of the leaves. On the other hand, the important pharmaceutical applications such as the use of the dried Aloe vera gel powder as an excipient in sustained release (SR) pharmaceutical dosage forms has to be explored further. The present study is development of water insoluble compound into a sustained release matrix tablets and the influence of Aloe vera gel powder in the dissolution and other physical properties of the SR matrix tablets were assessed. The HPMC and ethyl cellulose were used as polymer and different concentration of Aloe vera gel powder used as dissolution enhancer. Sustained release matrix tablets were formulated by direct compression method and subjected to various quality control studies as per the official pharmacopeial standards. The developed tablet formulation complies with the monograph. The results suggest that Aloe vera is improved the dissolution of curcumin. Dissolution kinetics suggests that all the formulation followed Korsemayer Peppas model via anomalous diffusion mechanism. To conclude the Aloe vera gel powder can be used as dissolution enhancer for improving the drug absorption of water insoluble drugs.
Keywords: Curcumin; Aloe vera ; Dissolution; Matrix tablets
The Aloe vera mucilage is well studied for its application in cosmetics. In the line the polysaccharide fraction present in the Aloe vera extract is finding new insights in the drug delivery technology. Aloe vera has a history of its use in folk medicine for skin and other disorders which goes back over thousands of years. Many scientific reports established the health beneficial effects of inner leaf gel and the high molecular weight polysaccharides attributes to the effects. Recent clinical studies have shown that use of Aloe vera improves the bioavailability of certain nutrients such as vitamin C and vitamin E by as much as 200%. Aloe vera is a biological vehicle in that it acts as a physical or physiological carrier for active biological agents but, also adds biological activity to the test agent [1-4]. In effect Aloe vera is a physical carrier as well as adds to activity [5-7]. The proposed work is established the application of natural Aloe vera polysaccharide as an excipient in formulation research. Curcumin the phytoconstituent in turmeric was formulated as sustained release matrix tablets using HPMC. Piperine and Aloevera was used in the formulation as solubility enhancers, the present investigation is focus in a view to use the freeze dried polysaccharide fraction of Aloe vera mucilage in development of sustained release formulation for water insoluble compounds. Specific object of the study is to develop sustained release matrix tablets of curcumin using HPMC and ethyl cellulose along with polysaccharide powder of Aloe vera and piperine, and to compare the solubility enhancement property of Aloe vera with Piperine. The bioavailabilty of curcumin can be improved by the developed formulation.
Curcumin and piperine was purchased from Sigma Aldec, Germany, Aloe vera gel powder was a gift sample from Sanjivani Phytopharma, Gujrat. HPMC K100 were purchased from SD fine chemicals, Mumbai and all other materials were of commercial grade.
Standard graph for curcumin
Calibration curve of Curcumin was constructed using Phosphate buffer pH 7.4 in the concentration of 1-10 μg/mL. The drug was analyzed spectrophotometrically (UV/VIS Spectrophotometer-JASCO; V-530) at 430 nm (regression coefficient r2=0.9999 in buffer pH 7.4)
Formulation development
Sustained release matrix tablets of curcumin were formulated using different ratio of HPMC K100 along with Aloe vera gel powder and piperine. The ratio of the polymer and other excipients are selected as per the trail batch results. Micro crystalline cellulose and Di calcium cellulose were added as direct compressible vehicles. Magnesium stereate and talc were chosen as lubricant. The powder blend is compressed into tablets by direct compression method using 12 mm punch in rotary tablet punching machine (Rimek Minipress 1).
Precompression evaluation
The powder blend is subjected to standard quality control studies as per the IP monograph protocols.
Tablet evaluation
Drug content analysis: The prepared tablets were weighed and powder equivalent to 50 mg of curcumin was accurately weighed and transferred into 100 mL standard flask and methanol is added to the flask. The mixture was then filtered and 1 mL of the filtrate was suitably diluted with methanol to obtain 100 μg/mL of curcumin ans analyzed for curcumin at 430 nm using UV-Visible Spectrophotometer (Shimadzu) keeping methanol as blank solution.
Degree of swelling: Curcumin matrice tablets were placed in dissolution baskets and kept at a pH 7.4 the weight of the tablets were noted at different time intervals up to 5 h. From the weight of swollen tablets and the weight of dry tablets the degree of swelling was estimated using the following formula
Swelling ratio Q=Vt/V0 (1)
where V0 and Vt are volumes.
Degree of swelling is typically expressed as the equilibrium swollen gel divided by the volume of the same gel before swelling.
Dissolution studies: In vitro drug release was studied using USP I apparatus, with 900 mL of dissolution medium maintained at 37 ± 1°C for 12 h, at 100 rpm. 0.1 N HCL (pH 1.2) was used as dissolution medium for the first 2 h, followed by Phosphate buffer pH 7.4 for further 10 h. 10 mL sample was withdrawn after each hour, and was replaced by an equal volume of fresh dissolution medium of same pH. Collected samples were analyzed colorimetrically at 430 nm, and cumulative percent drug release was calculated [8,9].
Kinetic analysis of dissolution data: The rate and mechanism of release of curcumin from the sustain release matrix tablets were analyzed by fitting the dissolution data into the zero-order equation [10]:
Q=k0t (2)
where Q is the amount of drug released at time t, and k0 is the release rate constant, fitted to the first order equation [11]:
ln (100–Q)=ln 100–k1t (3)
where k1 is the release rate constant. The dissolution data was fitted to the Higuchi’s equation [12]
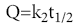
where k2 is the diffusion rate constant.
The dissolution data was also fitted to the well known equation (Korsmeyer equation), which is often used to describe the drug release behavior from polymeric systems [13]:
log (Mt/M∞)=log k+n log t (5)
where Mt is the amount of drug released at time t, M∞ is the amount of drug release after infinite time, k is a release rate constant incorporating structural and geometric characteristics of the tablet and n is the diffusional exponent indicative of the mechanism of drug release.
The prepared SR matrix tablets were evaluated for various physical properties. The densities for the powder blend of various formulations ranged between 0.28 ± 0.12 and 0.43 ± 0.21 g mL-1, as determined by the tapping method. The density results suggest that powder blend has good packing capacity. The carr’s index for all the formulations was found to be below 17%, indicates the desirable flow properties. The flow of the powder blend further assessed by determining the angle of repose for all the blends, it ranged between 25.82 ± 0.21 to 28.12 ± 0.08°. The value indicates desirable flow properties of the powder blend with HPMC K100 as matrix former.
All the batches of tablets were compressed under similar conditions to overcome the processing variables. Mass of the matrix tablets was 700 ± 8 mg, hardness was 6.1 ± 1.2 kg cm-2 and thickness was 3.1 ± 0.1 mm. The % friability of all the formulations was less than 1%. These results suggest that all formulations possess good handling properties. The drug content analysis in the SR matrix tablets was 96.8 ± 3.8%.
The FTIR spectrum of curcumin along with HPMC K100 showed a characteristic peaks for carbonyl group C=O stretching was observed in 1657 cm-1 (peak 6) and OH Stretching at 3450 cm-1 (peak 2). This confirms that the molecule under study, Curcumin has not undergone any structural changes. Thus no incompatibility issue is observed for Curcumin in the formulation (Figure 1). The degree of swelling was found to be better in Aloe vera formulations compared to piperine formulations.
The release profile of curcumin from the prepared formulations was analyzed by plotting time versus cumulative % release as shown in Figure 2.
Simple visual observation indicates that all the formulations showed a controlled release profile. Hydroxy propyl methyl cellulose K100 was used as the matrix former, HPMC K100 being a hydrophilic polymer should improve the hydrophilic nature of the surface of the tablets, previous reports suggests that HPMC K100 improves the solubility of curcumin. The study molecule curcumin is poorly water soluble which retarts the relese from matrix tablets. As the proportion of the HPMC increases the release process of curcumin decreases, while compared to formulations prepared with piperine, the Aloevera formulation shows a desired release profile for curcumin. The release profiles of the prepared formulations was found to be in the order of CA3>CP2>CP1>CA1>CP4>CP3>CA2. Hence, the formulation with low proportion of HPMC K100 along with Aloe vera mucilage freeze dried powder shows a desired release profile of 30% drug release in initial 2 h followed by a sustained release more than 10 h. The dissolution kinetic analysis for the formulation CA3, the calculated regression coefficient for higuchi, zero order and first order were 0.9821, 0.958 and 0.974 respectively. Therefore, the release seems to fit the higuchi model. To explore the release pattern of the CA3 formulation, the dissolution data is fitted to korsmeyer and peppas equation, which characterizes the transport mechanism. The value of the release exponent (n) for the optimized formulation was 0.7047, indicating release governed by anomalous diffusion which is nonlinear to the time. Similarly all other formulations dissolution was fitted to higuchi and korsemeyer peppas equation and the results are depicted in Table 1; Figures 3 and 4.
| Formulation | Higuchi | Korsemayer Peppas | Diffusion Mechanism | First Order | Zero Order | |
|---|---|---|---|---|---|---|
| r2 | r2 | n value | ||||
| CA3 | 0.9821 | 0.9969 | 0.7047 | Anomalous | 0.974 | 0.958 |
| CP2 | 0.881 | 0.9569 | 0.6856 | Anomalous | 0.856 | 0.969 |
| CP1 | 0.7696 | 0.9521 | 0.6023 | Anomalous | 0.741 | 0.875 |
| CA1 | 0.7968 | 0.9182 | 0.6591 | Anomalous | 0.835 | 0.936 |
| CA2 | 0.7921 | 0.7608 | 0.6627 | Anomalous | 0.848 | 0.922 |
| CP3 | 0.7402 | 0.7859 | 0.6485 | Anomalous | 0.819 | 0.908 |
| CP4 | 0.8238 | 0.8645 | 0.6879 | Anomalous | 0.9 | 0.96 |
Table 1: Dissolution kinetics of developed curcumin sustained release matrix tablet formulations.
The proposed work was carried to establish the influence of Aloe vera mucilage freeze dried powder in curcumin SR matrix tablets. The prepared SR matrix tablets with Aloe vera showed a initial burst release followed by a sustained release pattern.
The developed formulation is compared with the formulations prepared with piperine and the results are indicating that the Aloe vera powder improves the solubility without effecting the sustain release pattern of the curcumin formulation. The study implies that Aloe vera freeze dried powder can be used in the formulation of SR matrix tablets for water insoluble compounds. Further, IVIVC can be established using in vivo animal models to claim the potential use of Aloe vera powder as excipient in improving solubility of class II/IV drugs.