Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2025)Volume 15, Issue 2
Soluble fiber intake is essential in maintaining Glucose (Glc) homeostasis. However, the evidence attributes this effect only to viscous soluble fibers. Within the highly viscous and little explored fibers, is the Chia Mucilage (ChM), a seed present for centuries in our continent. The most accepted mechanism of action currently for viscous fibers is that they delay gastric emptying and intestinal transit, leading to better Glc management, but few studies have evaluated their action at the level of the intestinal barrier and the cells of this epithelium. Our objective was to evaluate the effect of ChM on Glc dialyzability and relative expression of SGLT-1 and GLUT-2. We performed in vitro static digestion assays for glucose and we measured Glc dialyzability and amylase activity, in the presence of ChM. Then, in Caco-2 cells challenged with Glc (25 and 35 mM) and ChM (1 mg/mL) for 2 or 12 hours, we determined mRNA relative expression of GLUT-2 and SGLT-1 by qRT-PCR and protein expression by Western Blot (WB). Digestion showed that in high Glc media, ChM leads to a decrease in Glc absorption, compared to inulin (as a control). We observed a time-dependent decrease in the residual activity of amylase in the presence of ChM, the effect was higher on pancreatic amylase than salivary amylase. There was a time-dependent effect on the relative expression of mRNA for GLUT-2 and SGLT-1, but without a decrease in protein expression. Our results showed that ChM has a potential adjuvant use in the prevention and treatment of glycemic alterations. We observed a decrease in intestinal-nutrient barrier contact; a decrease in the residual activity of amylase and/or an effect in the decrease of the expression of Glc transporters in the intestine, an aspect that requires more evidence.
Chia; Mucilage; Glucose; Soluble fiber; Goo; In vitro digestion; α-amylase; GLUT-2; SGLT-1
Type 2 Diabetes Mellitus (DM2) has increased dramatically and steadily over the last three decades, despite continuous worldwide efforts to prevent it. Western diet and sedentary lifestyle, leading to overweight and/or obesity, are considered its main risk factors. This disease, in addition to leading to many other health problems, carries a large economic burden for countries.
In order to reduce its prevalence, motivational interventions, modification of environments to promote behavioral changes, use of plants and drugs that improve blood parameters and promote weight loss have been proposed. Diets with modified carbohydrate and protein content, increased meal frequency and the effect of the Mediterranean and Paleolithic diets together with programmed physical exercise have been studied. The relationship of certain foods with the development of diabetes has also been studied, including dairy products, eggs, kiwi and nuts [1].
Soluble viscous dietary fibers, including psyllium, partially hydrolyzed guar gum and β-glucans from oats and barley, are associated with improvement of blood parameters related to DM2. Non-viscous soluble fibers of the fermentable type, especially inulin, have only shown improvement in intestinal dysbiosis and selective promotion of acetate- and butyrateproducing bacteria, metabolites that regulate inflammation and satiety. For insoluble fibers, there is no direct evidence implicating them in glycemic control. Fiber viscosity is involved in glycemic control and its long-term regular consumption decreases fasting blood glucose, insulin and glycosylated hemoglobin, in subjects at risk of developing DM2 and patients receiving treatment for DM2. The high viscosity of fiber would induce gastric distension, which delays gastric emptying and intestinal transit speed, allowing better handling of glucose loads. Even, in a model using IEC-6 cells, it was observed that β- glucans from oats induced a decrease in the rate of glucose absorption and in the expression of SGLT-1 and GLUT-2.
Chia seed (Salvia hispanica L.) was one of the main precolumbian crops in the Americas and was used for food, medicinal, artistic and religious purposes. Chia seed in an aqueous medium secretes a mucilaginous polysaccharide, which has been used as a thickening agent, foam stabilizer, emulsifier and surfactant. In addition, its consumption has been associated with improved glycemic and weight control, attributing this effect to the viscosity of its mucilage.
Our objective was to evaluate whether Chia Mucilage (ChM) decreases glucose dialyzability and the expression of its intestinal transporters SGLT-1 and GLUT-2 [2].
Extraction of ChM
Chia seed was obtained from the laboratory Benexia®. Four chia hydration tests were standardized with two temperatures (80°C and 25°C) and two grindings (whole and crushed seed). Omni mixer homogenizer (Omni Inc, Kennesaw GA, USA) was used to grind the seed and stirred mixture at 1300 rpm (RC-3B Sorvall Instruments, USA). Then, it was centrifuged at 4500 rpm, 0°C for 2 hours (RC-3B Sorvall Instruments, USA) and the mucilaginous phase was separated. Viscosity was measured with Brookfield RVF digital viscometer at 25°C and the results were expressed in mPas. The protocols were performed free of organic solvents and the determinations were repeated three times (Table 1).
| Assay | 1 | 2 | 3 | 4 | p* | |
| Semilla | Entera | Triturada | Entera | Triturada | ||
| Hydration | s/w Ratio | 1:20 | 1:20 | 1:20 | 1:20 | |
| Agitation | 1.300 rpm | 1.300 rpm | 1.300 rpm | 1.300 rpm | ||
| Time | 2 h | 2 h | 2 h | 2 h | ||
| Temperature (Tº) | 80ºC | 80ºC | 25ºC | 25ºC | ||
| Refrigeration | 24 h | 24 h | 24 h | 24 h | ||
| Extraction | Centrifugation | 4.500 rpm | 4.500 rpm | 4.500 rpm | 4.500 rpm | |
| Time | 2 h | 2 h | 2 h | 2 h | ||
| Temperature | 0ºC | 0ºC | 0ºC | 0ºC | ||
| Separation | Fase 2 | Fase 2 | Fase 2 | Fase 2 | ||
| Maintenance Tº | 4ºC | 4ºC | 4ºC | 4ºC | ||
| Seed yield (mg/g) | 5 | 3 | 2 | 5 | ||
| Viscosity (mPas) | 15 | 21 | 27 | 47 | <0.05 | |
Table 1: Chia Mucilage (ChM) extraction assay.
In vitro static and dialyzability digestion of glucose
The digestion protocol according to Minekus et al. was used. A semi-permeable dialysis membrane (diameter 16 mm) was incorporated in the intestinal stage to measure glucose influx at the end of intestinal digestion. The following assays were performed: a) 5 mM starch (Sigma, S9765. USA) (as control); b) ChM 25 or 50 mg/100 ml (ChM25 or ChM50) with 5, 10, 15 and 20 mM starch; c) Inulin (Inl; Orafti HPX® as control fiber) 1 or 2 g/100 ml (Inl1 or Inl2) with 5, 10, 15 and 20 mM starch.
The absorption process was calculated according to the formula: % absorption: (Final concentration of nutrient dialysate/initial concentration of food or solution) × 100. Glucose levels in the dialysis membrane and in final digest homogenate were measured with a glucose oxidase assay (BIO-RAD Laboratories). Amylase activity was measured during the oral (salivary amylase) and intestinal (pancreatic amylase) phases by the Dinitrosalicylic acid (DNS) method (Sigma; S2377. USA), using starch as substrate. Acarbose was used as positive control (Sigma; A8980. USA) [3].
Cell culture
The Caco-2 cell line (ATCC, HTB37, Manassas, Va, USA) was used as a model of intestinal epithelium. Cells were cultured at 37°C, 80% humidity and 5% CO2, in Dulbecco's Modified Eagle's Medium (DMEM, Sigma-Aldrich, St. Louis, USA), 10% Fetal Bovine Serum (FBS, PAA, Toronto, Canada) plus an antibiotic/antifungal mixture. Medium was changed every 3 days and cells were re-seeded every 7 days. Cells were used at passages 35-40, with normal growth and morphology, cultured in 6-well plates at confluence.
Cell viability assay
A cell viability assay was performed by MTT on Caco-2 cells cultured in increasing concentrations of ChM from 0.1 to 0.5 mg/ml, which were compared to an unexposed control. A 48- well plate and 8 replicates per concentration were used [4].
Measurement of the expression of GLUT-2 and SGLT-1 transporters in the presence of ChM
Caco-2 cells were cultured with basal DMEM medium (25 mM Glc) or with a glucose supplement (35 mM final) in the presence of ChM (50 μg/100 μL) for 2 or 12 hr. RNA extraction was then performed with Trizol Reagent (Invitrogen. USA). The extracted RNA was resuspended in RNase Free DNase Set (Qiagen. USA) and its concentration was determined spectrophotometric ally. RNA digestion for genomic DNA extraction was performed with the Turbo DNA-freeTM kit (ThermoFischer Scientific, Waltham, Massachusetts, USA) and the high-capacity cDNA reverse transcription kit (ThermoFischer Scientific, Waltham, Massachusetts, USA) was used for quantitative conversion of 2 μg total RNA to cDNA. Relative mRNA expression of GLUT-2 and SGLT-1 genes was performed using Brilliant II SYBR Green qPCR Master Mix (Agilent Technologies, Santa Clara, California, USA) on a Max Pro System 3000 (USA). Beta-2- Microglobulin (B2M) was used as the endogenous gene. The PCR amplification efficiency (range: 1.8-1.9) of each partition pair was calculated from the slope of the standard curve. The relative mRNA concentration was calculated using the ΔΔCt method. The partitions used were as follows (5' and 3' respectively): GLUT-2: GCCACACACTCACACACAAGACCTCCAAGGCCTGAAATTAGCCCA and SGLT-1: TCTCTCTGCAGCTCTCTACCGTCTTGCTCTCTCTGGGTCATAAGTCACC
To obtain a protein extract to the cell pellet 50 μL of lysis buffer with 1% protease inhibitor. It was then centrifuged at 14,000 rpm for 10 min and the supernatant was diluted in 50 μL of Tris Saline. Proteins were quantified by the Lowry method.
Subsequently, the expression of GLUT2 and SGLT2 transporters, was estimated by western blot, using as primary antibody anti GLUT2 (1:200) and anti SGLT-1 (1:200) (Abcam, Cambridge, UK) and as secondary anti IgG rabbit-HRP (1:1,000) antibody (Thermo Fisher Scientific, Massachusetts, USA). The bands were revealed by fluorescence scanning with luminol. β- actin (rabbit anti-β-actin monoclonal (1:2,000), Cell signaling technology, Massachusetts, USA) was used as a loading control [5].
Statistical analysis
Experiments were performed in triplicate and replicated at least 3 times on separate days. Statistical analysis was performed using Student's t-tests, Kruskal-Wallis with Dunn's post hoc test, and 2- way ANOVA with multiple comparisons. Significance was considered with p<0.05. Data are presented as mean ± Standard Deviation (SD) or median+Interquartile Range (IQR), depending on the normality of the variables. Analyses were performed using Graph Pad Prism 6.0 software [6].
Extraction and characterization of ChM
ChM was extracted using an organic solvent-free technique. The method that allowed the best seed yield (5 mg ChM/g seed) and the highest viscosity (47 mPas) (p<0.05, Kruskal Wallis), corresponded to model 4. Under these conditions a highly viscous solution was formed. Centrifugation allowed separation of the solution into 3 phases: Liquid surface, gelled intermediate and solid sediment. The gelled layer corresponding to unpurified ChM was stored. The ChM was oven dried (time and temperature), acquiring powder form. The solids content in this powder was 0.5 μg/μl. Upon rehydration the viscosity was completely recovered.
Effect of ChM on Glc dialyzability
The effect of ChM on the simple passage of glucose through the intestinal barrier was measured with an in vitro static digestion assay and a semi-permeable dialysis membrane was incorporated in the intestinal phase to measure the passage of glucose inside this membrane (Table 2). Inulin (non-viscous soluble fiber) was used as a control fiber at a concentration of 1 to 2 g, a dose at which it generates functional effects. We discarded the use of higher concentrations, since in solution it formed a very firm gel that was difficult to process [7].
| Fase | Oral | Gastric | Intestinal |
| Enzyme | Amylase 75 U/mL | Pepsin 2.000 U/mL | Pancreatin 100 U/mL |
| Bile salts | No | No | 10 mM |
| Fluid | KCl 15 mM | KCl 7 mM | KCl 7 mM |
| KH2PO4 3 mM | KH2PO4 1 mM | KH2PO4 1 mM | |
| NaHCO3 13 mM | 25 mM | NaHCO3 85 mM | |
| MgCl2 0.2 mM | NaCl 47 mM | NaCl 38 mM | |
| (NH4)2CO3 0.06 mM | MgCl2 0.1 mM | MgCl2 0.3 mM | |
| Sin NaCl | (NH4)2CO3 0.5 mM | Semipermeable membrane with pipes 0.1 M (pH 7.3) | |
| pH | 7 | 3 | 7 |
| Time | 2 min | 2 h | 2 h |
| Temperature | 37ºC (constant stirring) | ||
Table 2: In vitro static digestion protocol and dialyzability assay.
The percentage of Glc ingress in the presence of one digestion of normal medium and three digestions with medium high in glucose, in the presence of 1 and 2 grams/100 ml of inulin, is observed in Figure 1A. A decrease in Glc passage was observed, mainly in the presence of 2 grams of Inl (p<0.0006, 2-way ANOVA). For this reason, Inl2 is chosen as a control for ChM fiber in digestion experiments.
The dialyzability of Glc in a digestion in the presence of Inl2 and ChM (25 and 50 mg/100 ml), is observed in Figure 1B. These mucilage concentrations are equivalent to 5 and 10 grams/100 ml of whole chia seed according to the yield obtained (5 mg/g) and correspond to a recommended consumption portion. It was observed that, at a concentration of 5 mM glucose, both ChM concentrations have a lower effect than Inl2 in decreasing Glc passage (p<0.0001, Kruskal-Wallis). At concentrations between 10 and 20 mM glucose, ChM25 has a significantly lower effect than Inl2 (p<0.0001, Kruskal-Wallis), in reducing glucose uptake. ChM50 succeeds in reducing glucose passage to basal conditions of absorption, matching the effect of Inl2. At 20 mM glucose, it was observed that ChM25 showed a similar effect as Inl2 and ChM50 maintained complete inhibition of glucose passage, surpassing the effect of inulin (p<0.0001; Kruskal-Wallis) [8].
Since there is no recorded evidence of the inhibitory effect of ChM on α-amylase activity, which could be responsible for the observed effect, it was decided to measure α-amylase activity (%ARA) using the Dinitrosalicylic Acid (DNS) method (Figure 1C). By incorporating ChM in the in vitro digestion, we can observe that, both concentrations of ChM used inhibit salivary amylase activity, to a lesser but significant degree, compared to acarbose (inhibitor of both forms of amylase activity). On the other hand, pancreatic amylase was significantly inhibited by both concentrations of ChM, with respect to the control condition without exposure to ChM (p<0.0001, 2-way ANOVA and multiple comparison) [9].
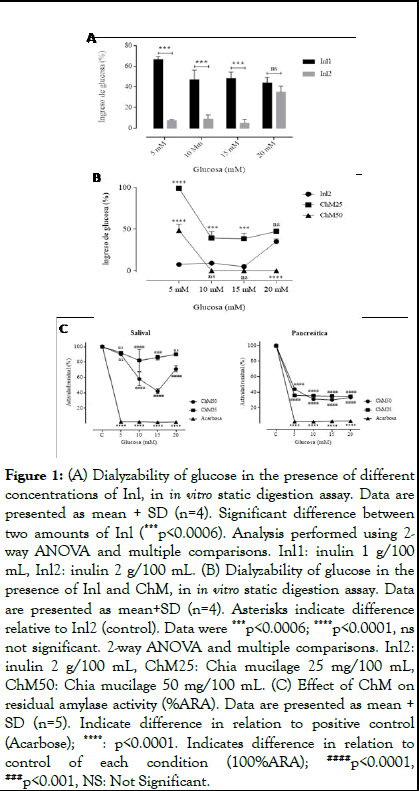
Figure 1: (A) Dialyzability of glucose in the presence of different concentrations of Inl, in in vitro static digestion assay. Data are presented as mean + SD (n=4). Significant difference between two amounts of Inl (***p<0.0006). Analysis performed using 2- way ANOVA and multiple comparisons. Inl1: inulin 1 g/100 mL, Inl2: inulin 2 g/100 mL. (B) Dialyzability of glucose in the presence of Inl and ChM, in in vitro static digestion assay. Data are presented as mean+SD (n=4). Asterisks indicate difference relative to Inl2 (control). Data were ***p<0.0006; ****p<0.0001, ns not significant. 2-way ANOVA and multiple comparisons. Inl2: inulin 2 g/100 mL, ChM25: Chia mucilage 25 mg/100 mL, ChM50: Chia mucilage 50 mg/100 mL. (C) Effect of ChM on residual amylase activity (%ARA). Data are presented as mean + SD (n=5). Indicate difference in relation to positive control (Acarbose); ****: p<0.0001. Indicates difference in relation to control of each condition (100%ARA); ####p<0.0001, ###p<0.001, NS: Not Significant.
Effects of ChM on relative expression of SGLT-1 and GLUT-2 in Caco-2 cells
In order to determine whether ChM exposure affected cell viability, MTT assays were performed by incubating Caco-2 cells with increasing concentrations of Chia mucilage (0.1 to 0.5 mg/ ml). There were no significant differences between the unexposed control and the concentrations used (p<0.9965, Kruskal Walis), thus Caco-2 cells maintained their viability intact. For the following experiments, 1 mg/ml ChM was used, which did not produce changes in cell morphology and growth [10].
Caco-2 cells were incubated with basal (25 mM glucose) or high glucose (35 mM) medium in the presence and absence of ChM and acute (2 h) and chronic (12 h) assays were performed. Subsequently, total mRNA was isolated and a protein extract was prepared to study GLUT-2 and SGLT-1 transporters by RTPCR and Western blot, respectively. No differences in the relative expression of GLUT-2 were observed in the acute stimulus (Figure 2A). However, at 12 hr of incubation, it was observed that incubation with ChM, reversed the increase in relative gene expression in high glucose medium (P<0.0001, Kruskal-Wallis test) (Figure 2B). Similar results were observed with the relative expression of SGLT-1 (Figures 2C and 2D). In chronic form, it is observed that ChM reverses the increase in relative SGLT-1 expression in high glucose medium (P<0.0001, Kruskal-Wallis test). No changes in the protein expression of GLUT-2 (Figures 3A and 3B) and SGLT-1 (Figures 3C and 3D) transporters were observed at any of the times and conditions studied.
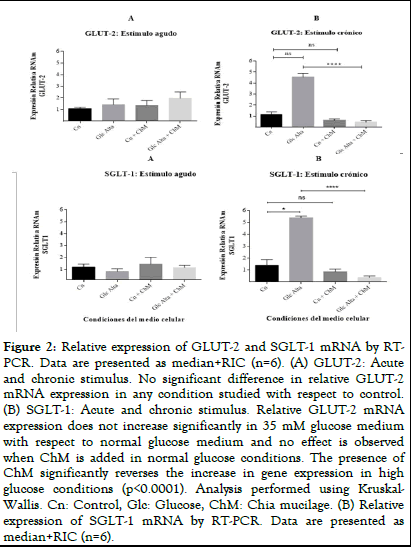
Figure 2: Relative expression of GLUT-2 and SGLT-1 mRNA by RTPCR. Data are presented as median+RIC (n=6). (A) GLUT-2: Acute and chronic stimulus. No significant difference in relative GLUT-2 mRNA expression in any condition studied with respect to control. (B) SGLT-1: Acute and chronic stimulus. Relative GLUT-2 mRNA expression does not increase significantly in 35 mM glucose medium with respect to normal glucose medium and no effect is observed when ChM is added in normal glucose conditions. The presence of ChM significantly reverses the increase in gene expression in high glucose conditions (p<0.0001). Analysis performed using Kruskal- Wallis. Cn: Control, Glc: Glucose, ChM: Chia mucilage. (B) Relative expression of SGLT-1 mRNA by RT-PCR. Data are presented as median+RIC (n=6).
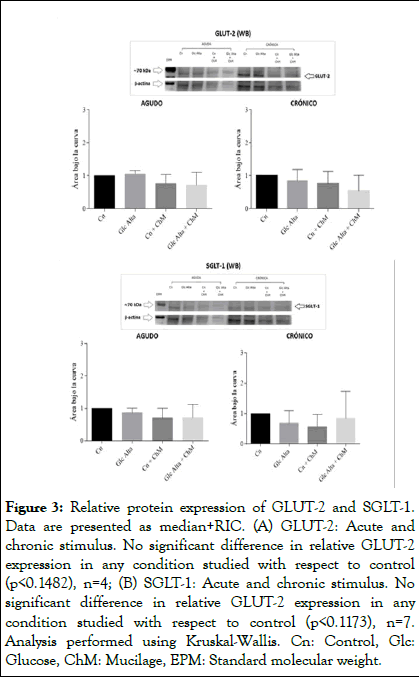
Figure 3: Relative protein expression of GLUT-2 and SGLT-1. Data are presented as median+RIC. (A) GLUT-2: Acute and chronic stimulus. No significant difference in relative GLUT-2 expression in any condition studied with respect to control (p<0.1482), n=4; (B) SGLT-1: Acute and chronic stimulus. No significant difference in relative GLUT-2 expression in any condition studied with respect to control (p<0.1173), n=7. Analysis performed using Kruskal-Wallis. Cn: Control, Glc: Glucose, ChM: Mucilage, EPM: Standard molecular weight.
Our study evaluated the effect of ChM at three levels: 1) Level of intestinal physical barrier for Glc entry, 2) Inhibition of α- amylase during the digestion process, 3) Expression of intestinal glucose transporters [11].
We were able to extract ChM using a technique free of organic solvents, a necessary requirement since these solvents can affect cell growth and are commonly included in extraction protocols in the food industry. The hydration time of 2 hours allowed complete water absorption, confirming what was observed in other protocols. Regarding the hydration ratio, some authors have used a ratio of 1:20, but others have obtained better results at ratios of 1:30 or 1:40. At room temperature, we obtained the highest viscosity when hydrating ground chia, unlike that reported by Muñoz-Hernández, who obtained better results at 80°C. Centrifugation allowed us to separate the mucilage from the seed with a seed yield of 5%. Muñoz-Hernández, obtained a yield of 7% by hydrating seed in a 1:40 ratio at 80°C, but controlling pH; Marín et al, used a 1:20 ratio at 25°C, just like our protocol, but extracted the ChM using pressure instead of centrifugation, obtaining a yield of 15.1%. Finally, Reyes- Caudillo et al., used an enzymatic-gravimetric AOAC assay extraction method obtaining a yield of 6.5%. All the comparative analyses shown should be viewed with caution, since the protocols used were different in relation to temperature control, pH, seed grinding or defatting or ChM separation method, however, it allows us to observe that the standards achieved by our separation method are within the reported values. Similarly, the hydration methodology for viscous fiber release has been used in a similar way for other fibers, such as psyllium husk (Figure 4) [12].
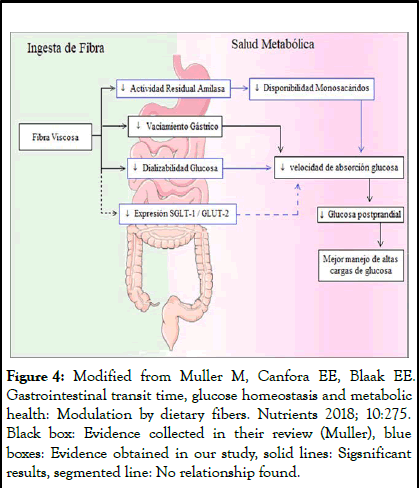
Figure 4: Modified from Muller M, Canfora EE, Blaak EE. Gastrointestinal transit time, glucose homeostasis and metabolic health: Modulation by dietary fibers. Nutrients 2018; 10:275. Black box: Evidence collected in their review (Muller), blue boxes: Evidence obtained in our study, solid lines: Sigsnificant results, segmented line: No relationship found.
Rehydration proved the versatility of its storage forms (gel or powder), which is added to other desirable characteristics, such as being a low-caloric compound with proven properties as a thickening agent, foam stabilizer, emulsifier and surfactant to stabilize emulsions, in addition to having shown that its viscosity is maintained at temperatures up to 80°C and that it has good stability in freezing and thawing. All of the above makes it a very attractive product for the food industry. ChM has already been successfully tested as a fat substitute in a cookie, achieving good acceptance and as a fat substitute in yogurts, maintaining consistency, firmness, creaminess and acidity. A recent study replaced the egg in a pork hamburger with chia seed gel and achieved improved texture, softness and chewiness, the products were perceived as juicier and softer than the controls and also slowed oxidative changes during storage. ChM was also tested as part of a coating for cold-preserved strawberries and an increase in shelf life was observed. An interesting study, as a clean label technology strategy, evaluated the properties of ChM as a phosphate substitute for low-fat sausages, showing a substitution of up to 50% of phosphates, with promising product acceptability. By way of reinforcing its multiple uses in the food industry, a study evaluated three methods of drying and subsequent rehydration, showing that ChM recovered all its properties and confirmed the adjustability of its viscoelastic behavior as a function of concentration. Undoubtedly, all of the above, together with its non-toxicity, biodegradability and digestibility, offer a promising alternative to thickeners already available on the market (polyvinyl alcohol, carrageenan and alginate) and to gastronomic innovation [13].
The main obstacle to its use is the impossibility of separating the ChM at home and the need for its industrial extraction, since without this process, the mucilage remains strongly adhered to the external cellular layer of the seed. In addition, the degree of processing affects its viscosity and as has been studied in guar gum, this results in a loss of glycemic control benefits. Therefore, it is essential to study its effects in different types of food matrices, for example in preparations that are exposed to high temperatures (baking), against heat/pressure extrusion methods (cereals), in different extraction protocols or against hydrolysis methods that are commonly applied in the food industry to improve product acceptability, but that affect viscosity [14].
Once our extract was characterized, we measured the passage of Glc through a dialysis membrane at the end of an in vitro digestion. All results indicated that in media high in Glc, ChM led to a decrease in Glc absorption, even greater than that observed with Inl, attributing this to its viscous condition. This result is supported by clinical studies, which have shown that the long-term effects of viscous fiber are proportional to the initial glycemic control, the effect being greater in people with DM2 and higher blood glucose levels than in people with Glc intolerance and showing no effect on euglycemia.
We also observed an effect on the decrease of %ARA, which is affected by the exposure time, since the effect is greater in pancreatic amylase than in salivary amylase. The only previous evidence found relating viscous fiber to the reduction of α- amylase activity was observed in an aqueous extract obtained from Auricularia polytricha (wood ear fungus). Although no studies were found relating fiber and amylase activity, there are studies of the α-amylase inhibitory effect of polyphenols, which include chlorogenic acid, florizin, epigallocatechin gallate, epicatechin and malvidin-3-glucoside (Mlv-3-glc). In addition, phenolic compounds isolated from Nigerian propolis (isoflavonoids, diarylpropane and prenylated flavanone) and phenols from green pistachio hulls (floroglucinol and gallic acid) were observed to have an inhibitory effect on both α-amylase and α-glucosidase. And there is also evidence of isoflavones from Potentilla astracanica and compounds obtained from cinnamon extract, including tannins, flavonoids, glycosides, terpenoids, coumarins and anthraquinones, which all showed α- glucosidase inhibitory activity [15]. Although this project did not include in its objectives the determination of polyphenol levels in chia seed, it is known that the main polyphenols it contains are chlorogenic acid, caffeic acid, quercetin and kaempferol and since ChM was not purified, it would be interesting to analyze its final composition in future research. According to the classical model, the effect on amylase would be attributed to reduced enzyme-nutrient binding due to increased viscosity (physical barrier), but direct enzyme inhibition cannot be ruled out, since studies of enzyme kinetics of polyphenols provide evidence of this action.
Although analyses are lacking, it is valuable to observe the important effects of ChM on glucose absorption and amylase activity at possible and recommended consumption doses, even more so if we consider that other in vitro assays give evidence that ChM retains its structure and viscosity throughout digestion and independent of its concentration, which gives lights that its viscous capacity is maintained under biological conditions. In addition, a new direction of study is opened: The effect of ChM on α-glucosidases, which were not evaluated in the present study [16].
Regarding cellular assays, Caco-2 cells showed a good response to ChM exposure, managing to maintain cell viability at concentrations up to 1 mg/ml. These results, are supported by studies of, who tested increasing concentrations of a chia seed extract and found a significant increase in cell viability when using 1 mg/ml. For the case of β-glucans from oats, IEC-6 cells have been exposed to concentrations of 4 to 6 mg/ml with a maintenance of cell viability. There is evidence indicating the presence of antioxidants in some chia extracts, which are associated with increased cell proliferation and reduced apoptosis, which could explain this effect.
When measuring the expression of transporters, the results obtained were inconclusive. An exposure-dependent effect on mRNA expression was observed for both transporters, with a significant decrease in their expression in high glucose medium only at 12 hours of stimulus, but no significant differences in relative protein expression were observed. Gene expression spans from DNA transcription to an RNAm until the mature protein has been localized in place and performs its function and in this process there are biological factors that affect. On the other hand, the small sample obtained for our study made it necessary to use a nonparametric statistical model, which is much more demanding, which can be noted by not finding statistical significance in the increase of Glc transporter expression in response to the stimulus of increased Glc in the medium, an increase that is biologically proven. In this case, the possible failure of the statistical model to explain biological aspects cannot be denied [17].
Scheme 1, modified from the team of Muller, seeks to incorporate the evidence obtained in our study, to explain actions of ChM. The mechanism proposed to date relates viscosity to a decrease in gastric emptying rate, which decreases the rate of glucose absorption, allowing better handling of high glucose loads. Our research showed that ChM, also leads to a decrease in glucose dialyzability and residual amylase activity and by this route also favors glycemic control. The observed effect suggests that there is a delay in Glc absorption mediated by this viscous fiber, but not a reduction in total Glc absorption, which is in line with other research showing that the total absorption rate does not decrease, as the slowing of gastric emptying stimulates the ileal brake, to attenuate nutrient loss. According to accumulated evidence, this delay in Glc absorption that improves glycemic control has not been observed in non-viscous and/or fermentable fibers, as is the case of Inl.
To our knowledge, this is one of the few studies that evaluates the effect of ChM at the level of the intestinal epithelium as a physical barrier and the first to investigate the effect on amylase activity and the expression of Glc transporters. For this study we used the intestinal cell line Caco-2, which is the most widely used, allowing us to have more evidence for a discussion of the results. The main advantage of intestinal cell models is their simplicity, repeatability and large-scale testing capacity, as well as being a cheaper alternative compared to animal models. In relation to ChM, although there are already viscous fibers used and that have been studied, our research provides evidence of a new viscous soluble fiber, which comes from a seed that is a fundamental part of the history of our continent and that for centuries was displaced and forgotten, which can be used as a coadjuvant treatment for prevention and treatment of glycemic disorders [18].
Regarding the limitations, we did not study the composition of the ChM, which could contain polyphenols or other antioxidants that evidence relates to a trophic effect or enzyme inhibition. Therefore, we can only conclude that some component present in this ChM is the one that generates this effect, but we cannot confirm which one or propose possible mechanisms of action. In addition, it would have been interesting to characterize the extract, to have evaluated different hydration ratios of chia and the viscosity obtained, as well as to evaluate different concentrations of the same extract, which would have allowed comparisons of the viscosity of ChM with other fibers and their associated effects. Despite its mentioned advantages, the Caco-2 cell line, being a one-dimensional model, is far from the in vivo environment and does not reflect the natural responses as well as the complex physiology of the intestine, which remains the advantage of using animal models [19].
In future research, it would be interesting to test the effect on the physical barrier directly in cells, especially using glucose markers, which would allow measuring the actual entry of glucose through the intestinal barrier. In addition, it would be interesting to study the kinetics over time (e.g. 6, 15 and 24 h) and to use different concentrations of ChM (e.g. 1, 2 and 3 mg/ ml). The use of transwell plates would allow studying the localization of transporters at different glucose loads in the presence and absence of ChM, especially GLUT-2, since there is evidence, although contradictory, that has observed transient recruitment of GLUT-2 to the apical membrane and there are few studies of the mechanism of GLUT-2-mediated glucose uptake in enterocyte cell culture models. Although the in vitro static digestion model used, has been well validated to study the digestion of individual substrates, an accurate prediction of in vivo bioaccessibility is limited as static models lack realistic enzyme substrate simulation in terms of ratios, pH profiles, transit times and elimination of digested products, in time and place. Given the above, it would be interesting to evaluate in vivo digestion including the presence of meals containing multiple substrates [20].
Our results show that ChM may have a functional effect by decreasing the availability of glucose for intestinal absorption, having a potential coadjutant use in the prevention and treatment of glycemic disorders. This effect could be attributed to: (i) The decrease in starch solubility due to the physical barrier effect provided by the viscosity of ChM; (ii) A possible effect on the decrease in residual amylase activity, which requires determining which specific component of the extract is responsible, and/or (iii) An effect on the decrease in the expression of glucose transporters in the intestine, an aspect that requires further evidence. It is essential to continue studies to delineate the mechanism of action of ChM on glucose absorption, adding in vivo digestion tests, measuring glucose absorption and testing the effect of different concentrations of ChM on glucose management. Given its promising benefits, continuing with its research and promoting its consumption is fundamental and also a way of vindicating the native crops of our continent.
The authors have declared that no competing interests exist.
Conceived and designed the experiments: MAO, MAG and AVA. Performed the experiments: AVA. Analyzed the data: AVA, MAG, MR and MAO. Wrote the paper: AVA, MAO, MR.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Valenzuelas A, Andrews M, Rivera M, Arredondo M (2025) Effects of Chia Mucilage on Intestinal Glucose Absorption: An In vitro Model in Intestinal Epithelial Cells. J Nutr Food Sci. 15:67.
Received: 27-Feb-2024, Manuscript No. JNFS-24-29824; Editor assigned: 01-Mar-2024, Pre QC No. JNFS-24-29824 (PQ); Reviewed: 15-Mar-2024, QC No. JNFS-24-29824; Revised: 14-Aug-2025, Manuscript No. JNFS-24-29824 (R); Published: 21-Aug-2025 , DOI: 10.35248/2155-9600.25.15.67
Copyright: © 2025 Valenzuelas A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.