Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Research Article - (2023)Volume 8, Issue 11
Assessment for presence and quantification of pesticide levels in fruits and vegetables that are among the main food sources is vital. Studies of this nature allow for gauging the extent of potential contamination and inform measures for human health preservation. We sought to establish the residual levels of imidacloprid in fruits and vegetables with reference to their dietary intake assessment in Amman, Jordan. Three hundred samples of local fruit and vegetables from Amman (Jordan) have therefore been collected for imidacloprid analysis in this study. The 1993 Placke and Weber method was use in the extraction and oxidation of imidacloprid including its derivatives into these samples and subsequent quantification by GC-MS was performed. Imidacloprid was found in 119 (39.7%) of the examined samples. The peak concentration (1.30 mg/kg) was found in an eggplant sample. All fruit and vegetable samples, except apricot, carrot, peaches, and okra samples had detectable imidacloprid levels. Green beans and banana had at least in three samples positive for imidacloprid, while all eggplant samples were imidacloprid positive. We also found that 8.3% of the analyzed samples had concentrations exceeding the Codex MRL whereas 2.7% of these samples had concentrations higher than the Canadian MRL. Although high imidacloprid residues were detected in samples of banana, eggplant and watermelon, their dietary intake assessment quantities were within limits that are considered as safe.
Insecticide; Imidacloprid; Food safety; Jordan
The use of insecticides boosts agricultural production by eliminating/suppressing tissue eating and sap sucking pests such as locusts (Schistocerca gregaria) and aphids (Aphidoidea spp). Extreme and disproportionate use of insecticides can contaminate freshwater as well as soil and farm products [1]. Annually, approximately 2.5 million tonnes of insecticides are applied globally [2]. In Jordan, average frequency of pesticide application is about ten times per growing season [3]. Out of the total insecticide applications, 27% are being applied on vegetables and fruits [4].
The most popular ones are neonicotinoid insecticides which consist of seven commercially marketed active ingredients (imidacloprid, acetamiprid, nitenpyram, thiamethoxam, thiacloprid, clothianidin and dinotefuran) [5]. Within this neonicotinoid pesticide group, imidacloprid (C9H10ClN5O2) is the second most widely used insecticide globally. Imidacloprid has remarkable activity at minimal application rate, minimal toxicity, superb systemic properties, and a long-lasting action towards controlling pests. Furthermore, imidacloprid is a systemic pesticide with translaminar action; it works as an antagonist of postsynaptic nicotinic acetylcholine receptors [6].
To warrant consumer safety as well as standardize global exchange, government agencies and the European Union Commission (EUC) have mandated Maximum Residue Limits (MRLs) for pesticides in food [7]. The MRL is the maximum quantity of a pesticide residue legally permitted in food. This quantity is usually attainable when pesticides are applied appropriately in accordance with Good Agricultural Practices (GAPs) [8].
Dietary intake assessment of fruit and vegetables is essential in order to ensure that insecticide residues are below their MRL. A strategy to evaluate the current scenario is the contrast between the Acceptable Daily Intake (ADI) and Maximum Permissible Intake (MPI) quantities of insecticide residues. In order to ensure safe consumption limits of fruits and vegetables with regard to the exposure of insecticide residues, dietary intake consideration is needed. Therefore, the objective of this study was to determine residual quantities of imidacloprid in fruits and vegetables as well as establishing their dietary intake in Amman, Jordan.
Sampling
A total of 300 fruits and vegetables samples were collected between 2016 and 2018. Fruit and vegetables 15 samples of each (apples, bananas, grape, apricot, peaches, watermelon, cantaloupe, strawberry, carrot, tomato, cucumber, potatoes, eggplant, zucchini, cauliflower, cabbage, bell pepper, green beans, okra and spinach), representative of generally consumed produces in Jordan were purchased from local markets in Amman, Jordan. To avoid contamination and deterioration while transporting to the laboratory for analysis, all samples (1-2 kg each) were collected in sterile plastic bags, labeled, and placed in an icebox.
Apparatus
• Gas Chromatography-Mass Spectrometry (GC-MS)
• Rotary evaporator, Büchi-B-721(Büchi, Switzerland)
• Blender/mixer (Warring product division, America)
• Electronic balance (sensitivity ± 0.0001)
Chemicals and reagents
The following solvents and reagents were used for pesticide residue analysis; 99% 6-chloronicotinic acid (C6H4ClNO2), 97% sodium hydroxide (NaOH), 99% anhydrous sodium sulfate (Na2SO4), 99% potassium permanganate (KMnO4), sodiumbisulfite (NaHSO3), 99% t-butylmethyl ether (C5H12O), Amberlite XAD-4 (20-60mesh), and N-methyl-N- (trimethylsilyl) trifluoroacetamide (C6H12F3NOSi) were procured from a Germany company (Sigma-Aldrich). Methanol (99.9%) and acetonitrile (99.9%) were bought from Merck, (Germany). Analytical standard imidacloprid was purchased from Bayer, (Germany). Gases (Nitrogen and Helium) (99.999%) gas were obtained from Amman LPG Filling Gas Station, Jordan. The organic-free deionized water used was obtained from Riedel de- Hean (Germany).
Sample extraction
Approximately 1000 g sample of fruit/vegetable were blended to slurry. Fifty grams of the slurry were immersed in methanol:water (75% v/v, 300 ml) for 1800 s. The mixture was then shaked for homogenized and filtered, after which the filtrate was made with methanol up to 500 ml. A 100 ml aliquot was evaporated to 20 ml using the rotary evaporator at 60°C, then transferred to a pre-conditioned column packed with XAD-4 resin (for water samples, 250 ml aliquot were concentrated to 20 ml and transferred to the column). The column was washed two times with methanol:water (75% v/v, 20 ml), after which the retained compounds were eluted with methanol (100 ml) and a vacuum rotary evaporator was used to concentrate the compounds into to 1 ml then to drying using a gentle stream of nitrogen.
Extraction and analysis of imidacloprid
Imidacloprid and its variants in the samples were isolated and oxidized using the method of Placke, et al. [9] to 6- chloronicotinic acid.
Samples oxidation
Using the following approach, imidacloprid and other variants of 6-chloropicolyl moieties were extracted in 1 ml concentrate were oxidized to 6-chloronicotinic acid: The concentrate was diluted in 100 ml of water before adding oxidizing solution (32% NaOH and KMnO4 (5%, 50 ml-100 ml added to samples of hops)). The mixture was refluxed by with stirring for 5 minutes and water (50 ml) was added. The flask was cooled to 15°C under with agitation in an ice bath (10 minutes). Sulfuric acid (10%, 50 ml) was added, followed by 3 g additions of solid sodium bisulfite with cooling and agitation. To maintain a pH value of ≤ 1, more sulfuric acid was added. Subsequently, a solution of 150 ml t-butylether was used for final extraction with the organic phase filtered through 30 g anhydrous sodium sulfate and dried in an evaporator.
Sample derivatization
Acetonitrile (2 ml) was used to dissolve the extract and a 250 μl aliquot of the solution was derivatized to 6-chloronicotinic acid trimethylsilyl ester through vigorous mixing with N-methyl-Ntrimethylsilyl- trifluoroacetamide (MSTFA). A1 μl aliquot was injected into the GC/MS under split-less mode.
GC/MS working conditions
• Agilent 6890 series II, with auto sampler 7683 series.
• Detector: Mass selective quadrupole-detector, Agilent 5973N, electron impact ionization, 70 eV.
• DB-5 column (5% phenylmethyl polysiloxane polymers), 30 m × 0.25 mm i.e., 0.25 μm film thickness.
The analysis of imidacloprid in samples was carried out using an Agilent 6890 series II GC, with auto sampler injector 7683 series equipped with mass selective quadrupole detector, and DB-5 capillary column. Helium (99.999%) was the carrier gas running at flow rate of 1 ml/min. The splitless mode was used for sample injection (1 μl) (injector temperature at 260°C). The oven temperature started at 100°C (1 minute), 100°C-180°C (rate: 15°C/min), then raised to 300°C (rate: 30°C/min), and held for 3 minutes. The tow ions used in quantification of 6- chloronicotionic acid-trimethylsilyl ester were 214 as the target ion and 170 as the qualifying ion.
Recovery tests and detection limits
Standard solutions of 6-chloronicotinic acid were derived according to the above-mentioned protocol and their aliquots were injected prior to of the sample aliquots. The validated analytical method was used [9]. Some quality control parameters were confirmed and found to be similar to the results of [9]; the MDL standard mixture after dilution several times (triplicate standard deviation of a blank generated by similar abovementioned method) displayed results of detection limits ranging from 0.015 to 0.05 mg/kg, and the spike recovery was 74-106%.
The highest number of samples detected as having residual imidacloprid was recorded in eggplant and zucchini (Cucurbita pepo) (100% and 80%, respectively). Bananas and green beans recorded the least number of samples having detectable residual imidacloprid (20%). There was no detectable imidacloprid in apricot (Prunus armeniaca), peaches (Prunus persica), carrot (Daucus carota subsp. sativus) and okra (Abelmoschus esculentus) (Table 1).
| Fruit/Vegetable | Mean | Range | Samples analyzed | Samples detected |
|---|---|---|---|---|
| Apples | 0.25 ± 0.21 | 0.19-0.83 | 15 | 9 (60%) |
| Banana | 0.04 ± 0.05 | 0.15-0.25 | 15 | 3 (20%) |
| Grapes | 0.07 ± 0.09 | 0.08-0.32 | 15 | 6 (40%) |
| Apricot | n.d. | - | 15 | 0 |
| Peaches | n.d. | - | 15 | 0 |
| Watermelon | 0.12 ±0.18 | 0.06-0.56 | 15 | 7 (46.7%) |
| Cantaloupe | 0.18 ± 0.19 | 0.07-0.61 | 15 | 9 (60%) |
| Strawberry | 0.10 ± 0.08 | 0.09-0.33 | 15 | 7 (46.7%) |
| Carrot | n.d. | - | 15 | 0 |
| Tomato | 0.11 ± 0.23 | 0.07-0.61 | 15 | 5 (33.3%) |
| Cucumber | 0.18 ± 0.18 | 0.06-0.62 | 15 | 11 (73.3%) |
| Potatoes | 0.05 ± 0.13 | 0.07-0.34 | 15 | 4 (26.7%) |
| Eggplant | 0.40 ± 0.33 | 0.08-1.30 | 15 | 15 (100%) |
| Zucchini | 0.18 ± 0.15 | 0.06-0.56 | 15 | 12 (80%) |
| Cauliflower | 0.18 ± 0.18 | 0.09-0.62 | 15 | 8 (53.3%) |
| Cabbage | 0.07 ± 0.10 | 0.09-0.36 | 15 | 5 (33.3%) |
| Bell pepper | 0.12 ± 0.12 | 0.06-0.42 | 15 | 8 (53.3%) |
| Green beans | 0.03 ± 0.14 | 0.06-0.34 | 15 | 3 (20%) |
| Okra | n.d. | - | 15 | 0 |
| Spinach | 0.09 ± 0.13 | 0.06-0.41 | 15 | 7 (46.7%) |
| Total | 300 | 119 (39.7%) | ||
| Note: n.d.=pesticide residue not detected. | ||||
Table 1: Residual imidacloprid in Jordan fruits and vegetables in mg/kg.
Various studies emphasize the significance of the different procedures (cutting, washing and heating) in food processing to reduce pesticide residues [10,11]. Related residual pesticide reductions to hydrolysis, enzymatic and redox reactions [12]. Washing fruits and vegetables is the most effective method towards removal of contaminating insecticide residues to minimize human ingestion. Cutting, sealing and pasteurizing have also been shown to generate gradual decreases in residue levels [13]. In the preparation of pomegranate (Punica granatum L.) juice, it has also been reported that the highest percentage of water-soluble pesticides are retained in the pulp [14].
Stone fruits (apricot and peaches), carrot and okra did not show imidacloprid residues (Figure 1). This can be attributed to physiological portioning of the insecticide to the photosynthesizing parts rather than the economic parts of the plant. High imidacloprid in eggplant, cucumber, cantaloupe, zucchini and apple (60-100%) can be explained by high physiological partitioning coefficient of insecticide to the economic parts of the plant which is the fruit. The physicochemical properties of imidacloprid determine its fate in different plant/crop species [15]. Imidacloprid in plants can be absorbed, stored, metabolized, and/or discharged to the environment. These progressions determine both the pesticide's impact on the plant as well as the characteristics of its residues.
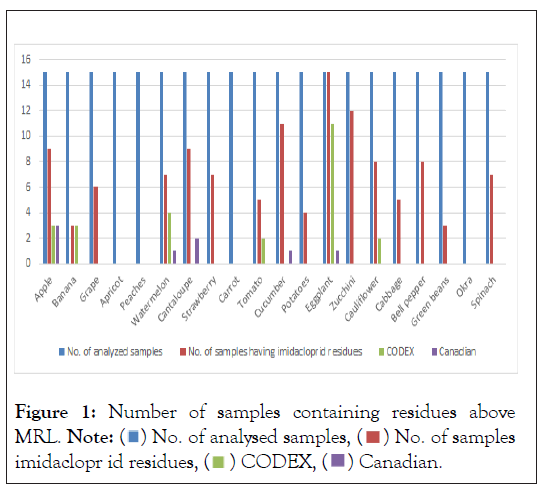
Figure 1: Number of samples containing residues above
MRL. Note:  No. of analysed samples,
No. of analysed samples,  No. of samples
imidaclopr id residues,
No. of samples
imidaclopr id residues,  CODEX,
CODEX,  Canadian.
Canadian.
The Codex pesticides residues database contains Codex Maximum Residue Limits (MRL) for pesticides and Extraneous Maximum Residue Limits (EMRL) adopted by the Codex Alimentarius Commission [16,17]. The foods listed in the Codex database must not exceed the MRL or EMRL (in mgkg-1) of the pesticide residue at (i) point of entrance into a nation [18] or (ii) point of entry into trade channels within a country [19].
According to our findings the Codex of imidacloprid for eggplant (0.20 mg/kg) were exceeded by the mean values 0.40 mg/kg recorded in Jordan (Table 2). The mean concentrations of imidacloprid recorded in Jordan for apples, Banana, Watermelon, grapes, strawberry, tomato, cucumber and potatoes were below the Codex standard values (Table 2). As shown in Table 3 and 4 is the comparison of imidacloprid levels among selected Asian countries. The data exposes that apples in Jordan had the highest mean imidacloprid levels (0.25 mg/kg) relative to other Asian countries; this can be attributed to non-adherence to GAPs and intensive insecticide usage in the Jordan apple agricultural industry [20,21].
| mg/kg | ||||||
|---|---|---|---|---|---|---|
| Fruit/ vegetables | Codex MRL (20) | With residue<Codex MRL | Canadian MRL (21) | With residue<Codex MRL | Mean Max | |
| study | ||||||
| Apple | 0.5 | 3 | 0.6 | 3 | 0.25 | 0.83 |
| Banana | 0.05 | 3 | - | - | 0.04 | 0.25 |
| Grape | 1 | 0 | 1.5 | 0 | 0.07 | 0.32 |
| Apricot | - | 3 | 0 | n.d. | ||
| Peaches | 0.5 | 0 | 3 | 0 | n.d. | |
| Watermelon | 0.2 | 4 | 0.5 | 1 | 0.12 | 0.56 |
| Cantaloupe | - | 0.5 | 2 | 0.18 | 0.61 | |
| Strawberry | 0.5 | 0 | 0.5 | 0 | 0.1 | 0.33 |
| Carrot | - | - | 0.4 | 0 | n.d. | |
| Tomato | 0.5 | 2 | 1 | 0 | 0.11 | 0.61 |
| Cucumber | 1 | 0 | 0.5 | 1 | 0.18 | 0.62 |
| Potatoes | 0.5 | 0 | 0.4 | 0 | 0.05 | 0.34 |
| Eggplant | 0.2 | 11 | 1 | 1 | 0.4 | 1.3 |
| Zucchini | - | - | - | - | 0.18 | 0.56 |
| Cauliflower | 0.5 | 2 | - | 0.18 | 0.62 | |
| Cabbage | 0.5 | 0 | - | 0 | 0.07 | 0.36 |
| Bell pepper | 1 | 0 | 0.12 | 0.42 | ||
| Green beans | 2 | 0 | - | - | 0.03 | 0.34 |
| Okra | - | - | 1 | 0 | n.d. | |
| Spinach | - | - | 3.5 | 0 | 0.09 | 0.41 |
| Note: MR=Maximum Residue Limits and n.d.=pesticide residue not detected. | ||||||
Table 2: The Codex and Canadian imidacloprid maximum residue limits in fruits and vegetables and number of samples above MRL.
| Fruit/Vegetables | Palestine (mean) (28) |
Kuwait (range) (29) |
Pakistan (mean) (30) |
India (range) (31) |
Jordan (mean) (max) study |
|
|---|---|---|---|---|---|---|
| Apple | 0.24 | 0.2-0.65 | 0.02-0.26 | 0.25 | 0.83 | |
| Banana | 0.18 | n.d.-0.04 | 0.04 | 0.25 | ||
| Grape | 0.08 | n.d.-0.98 | 0.04-0.78 | 0.07 | 0.32 | |
| Apricot | n.d. | |||||
| Peaches | 0.36 | n.d. | ||||
| Watermelon | 0.32 | n.d.-0.23 | 0.12 | 0.56 | ||
| Cantaloupe | 0.18 | 0.61 | ||||
| Strawberry | n.d.-0.2 | 0.1 | 0.33 | |||
| Carrot | n.d. | n.d. | ||||
| Tomato | n.d.-0.51 | 0.24-0.46 | 0.11 | 0.61 | ||
| Cucumber | 0.11 | 0.05-1.2 | 0.18 | 0.62 | ||
| Potatoes | 0.4 | n.d. | 0.19-1.32 | 0.05 | 0.34 | |
| Eggplant | 0.41 | n.d.-0.09 | 0.81 | 0.13-0.21 | 0.4 | 1.3 |
| Zucchini | 0.18 | 0.56 | ||||
| Cauliflower | 0.26 | 0.29-0.93 | 0.18 | 0.62 | ||
| Cabbage | n.d. | 0.08-0.89 | 0.07 | 0.36 | ||
| Bell pepper | n.d.-0.01 | n.d.-0.45 | 0.12 | 0.42 | ||
| Green beans | 0.1 | 0.03 | 0.34 | |||
| Okra | 0.49 | n.d.-0.11 | n.d. | |||
| Spinach | 0.09 | 0.41 | ||||
| Note: n.d.=pesticide residue not detected. All values are in mg/kg. | ||||||
Table 3: Mean and range for imidacloprid in Asian countries.
| Fruit/Vegetable | Mean µg/kg | Mean annual intake of commodity per person (kg) (F) (Amman) | EDI (µg/kg bw daily) | Hazard index |
|---|---|---|---|---|
| Apples | 250 | 7.37 | 0.084 | 0.14 |
| Banana | 40 | 10 | 0.018 | 0.03 |
| Grapes | 70 | 2.87 | 0.009 | 0.015 |
| Apricot | 0 | - | 0 | 0 |
| Peaches | 0 | - | 0 | 0 |
| Watermelon | 120 | 7.94 | 0.043 | 0.072 |
| Cantaloupe | 180 | 2.44 | 0.02 | 0.033 |
| Strawberry | 100 | 0.49 | 0.002 | 0.003 |
| Carrot | 0 | - | 0 | 0 |
| Tomato | 110 | 28.69 | 0.144 | 0.24 |
| Cucumber | 180 | 14.42 | 0.118 | 0.197 |
| Potatoes | 50 | 18.24 | 0.042 | 0.069 |
| Eggplant | 400 | 6.58 | 0.12 | 0.2 |
| Zucchini | 180 | 4.64 | 0.038 | 0.063 |
| Cauliflower | 180 | 4.26 | 0.035 | 0.058 |
| Cabbage | 70 | 1.73 | 0.006 | 0.01 |
| Bell pepper | 120 | 3.2 | 0.017 | 0.028 |
| Green beans | 30 | 0.44 | 0.001 | 0.001 |
| Okra | 0 | - | 0 | 0 |
| Spinach | 90 | 1.03 | 0.004 | 0.007 |
Table 4: Calculation of estimated daily intake and hazard index of imidacloprid in different fruits and vegetables.
Imidacloprid (Figure 2) is a systemic insecticide that can be taken up by plants from soil or through leaves. It can spreads through plant's stems, leaves, flowers and fruits [22]. Insects that chew/suck treated plants are susceptible to ingesting imidacloprid, causing it to damage their nervous systems and eventual death.
Figure 2: Chemical structure of imidacloprid.
People can also be exposed to chemicals through (i) skin contact, (ii) inhalation and (iii) ingestion. Since imidacloprid is a systemic insecticide, exposure to this insecticide can occur upon eating fruit and vegetables that were grown in fields treated with imidacloprid (Table 4). Although imidacloprid is not easily penetrable to the skin, when ingested, it can cross the lining of the stomach and ultimately moves through the bloodstream, spreading to various anatomical sites. Imidacloprid can be metabolized by the liver and excreted through faeces and urine [23]. This is supported by Wang, et al, [24,25] who showed that rats fed with imidacloprid, excreted 90% of the dose within 24 hours.
Based on animal studies, the Environmental Protection Agency (EPA) concluded that the carcinogenicity of imidacloprid is unsubstantiated. Moreover, the International Agency for Research on Cancer (IARC) has also not classified imidacloprid as potentially carcinogenic. However, studies which administered imidacloprid to pregnant rats and rabbits, demonstrated adverse reproductive effects, including offspring with reduced skeletal development [26-31]. The doses that caused the problems in the pups were toxic to the mothers. Currently, the developmental or reproductive effects of imidacloprid in humans remain understudied.
In conclusion, it is necessary to consider the advantages of using insecticides in agriculture to produce healthier crops, while contemplating the probable health risks associated with potentially poisonous insecticide residues in food. Although higher imidacloprid residues were detected in some samples of banana, eggplant and watermelon collected in Amman Jordan, their dietary intake assessment levels were found to be within safe limits. Good Agricultural Practices (GAPs) following the controlled and proportionate application of insecticides must be embraced. In addition, household fruit and vegetable preconsumption processing practises like washing, peeling, and cooking can significantly lower the contaminating pesticide residues to below MRL values.
Citation: Al Hawadi JS (2023) Determination of Imidacloprid Residues in Fruits and Vegetables from Amman (Jordan) Using Gc-Ms. J Food Microbial Saf Hyg. 8:208.
Received: 01-May-2023, Manuscript No. JFMSH-23-23721; Editor assigned: 03-May-2023, Pre QC No. JFMSH-23-23721; Reviewed: 17-May-2023, QC No. JFMSH-23-23721; Revised: 24-May-2023, Manuscript No. JFMSH-23-23721; Published: 31-May-2023 , DOI: 10.35248/2476-2059.23.8.208
Copyright: © 2023 Al Hawadi JS. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.