Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2013) Volume 3, Issue 2
Amurca (olive oil lees) is one of olive oil byproducts which is a watery bitter tasting and dark colored sediment that settles at the bottom of crude olive oil containers over time. In this study, gross composition, total phenolic compounds, antioxidant activity, HPLC profile of phenolic compounds, lipid peroxidation inhibitory activity, free fatty acid and peroxide values of amurca were determined. The gross composition of Jordanian amurca was as follows: carbohydrates 0.74 ± 0.02%, proteins 0.7 ± 0.02%, fats 49.43 ± 0.29%, moisture 47.33 ± 0.30%, ash 0.89 ± 0.05% and fiber 0. 92 ± 0.03%. Total phenolic compounds content was 289 mg GAE/100 g of amurca and antioxidant activity was 22.3 ± 0.21 mg vitamin E equivalent/100 g. Peroxide value was 1.78 ± 0.03 meqO2/kg amurca, free fatty acid value was 1.62 ± 0.029 (oleic acid%) and LPO inhibition was 95.7%. The most abundant phenolic compounds detected by HPLC were oleuropen, gallic acid, 3-hydroxyphenol, sinapic acid, kaempherol, isopropyl-5-methyl phenol and luteolin.
Keywords: Jordanian amurca; Gross composition; Total phenolic compounds; Antioxidant activity; HPLC of phenolic compounds; Lipid peroxidation; Free fatty acid value; Peroxide value
Olive oil is the major product of pressed olive fruits. Mechanical extraction of oil involves crushing whole olive fruits, kneading the resulting paste, pressing, separating, collecting free flow oil and finally separating amurcafrom olive oil via centrifugation and filtration [1].
Amurca is known as olive oil lees in english [2] and Turtub in Jordan. Although some inconsistency is found in the literature concerning the use of the term amurca; in this article the term amurcarefers to watery bitter tasting and dark colored sediment that settles at the bottom of crude olive oil container over time [1,2]. Olive oil content of amurca varies from 12-460 mg/kg oil depending on the type of mills [3]. Jordan institution for Standards and Metrology did not specify amurcacontent in Jordanian olive oil and since the Jordanian consumer prefers the flavor and aroma of amurca, then Jordanian olive oil produced for local consumption contains variable amounts of amurcaand the final step of filtration is skipped [4].
Historically, amurcahad several uses such as: herbicide, pesticide and for oiling leather [1,2,5]. Bitler et al. (2005) [6] reported that vegetation water decreased tumor necrosis factor (α-TNF) production and anti-inflammatory activity in the mouse. Furthermore, Bitler et al. (2007) [7] reported a decrease in pain and inflammation in patients with osteoarthritis and rheumatoid arthritis supplemented with vegetation water; this has been explained by the presence of strong antioxidant activity in amurca [7].
The composition of Jordanian amurcawas not studied earlier. So, this study was designed to study gross composition, total phenolic compounds; antioxidant activity, HPLC profile of phenolic compounds, lipid peroxidation inhibitory activity, free fatty acid and peroxide values of amurca.
Amurca samples
Amurca samples were obtained from olive oil bought from an olive oil mill in the northern Jordan. Amurca was extracted from olive oil after 12 months of storage by centrifugation at 4000 rpm (1252xg) for 30 minutes and stored at -18°C until use.
Gross chemical analysis
Amurca gross composition was determined according to the approved Association of Official Analytical Chemists [8].
Preparation of amurcaextracts: Fifty grams of amurcasample were diluted in 50 mL of hexane and the mixture was washed three times with 30 mL of methanol/water mixture (60:40). The mixture was shaken for 2 min before allowing the two phases to separate in a separator funnel. The methanolic extracts were then washed with 50 mL of hexane and finally brought up to 100 mL in a volumetric flask and stored at -18°C until use [9].
Total phenolic compounds
Total phenolic compounds of amurcaextracts were determined according to the Folin-Ciocalteu procedure adapted from Hajimahmoodi et al. [10]. Gallic acid was used as calibration standard and results were expressed as mg gallic acid equivalent (mg GAE /100 g fresh weight).
Antioxidant activity
Antioxidant activity of amurcaextracts were determined spectrophotometrically using Fe3+ reducing antioxidant power assay (FRAP) [11]. For construction of the calibration curve, six concentrations of vitamin E (4, 6, 8, 10, 15 and 20 mg) were used and results were calculated as mg vitamin E equivalent (mg vitamin E/100 g fresh weight).
Free fatty acid and peroxide values
Oil extraction from amurca: Oil was extracted from amurcaby soaking amurcasamples in hexane (1:3) over night, the mixture was centrifuged at 3000 rpm (723xg) for 20 minutes and the supernatant was collected, and hexane was evaporated using rotary evaporator, then free fatty acid and peroxide values were determined according to the official EU method [8,12].
Determination of lipid peroxidation: Lipid peroxidation was determined by measuring the concentration of malondialdehyde in the liver homogenates according to Ohkawa et al. [13] and Lin et al. [14]. Amurca extracts (hexane, methanol, water) were prepared according to method described by Favati et al. [9]. The inhibition percent of nonenzymatic LPO induced by Fe2+/ascorbate mixture was determined according to the following equation [15].
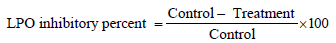
Where,
Control=absorbance of MDA/TBA complex formed in the absence of amurcaextract.
Treatment=absorbance of MDA/TBA complex formed in the presence of amurcaextract.
Determination of phenolic compounds by HPLC
Extraction method: Phenolic compounds were isolated from amurcaby extraction with petroleum ether. The extract was then washed with 60 mL of methanol/water mixture (60:40) 3 times. The 3 aliquots were combined and washed with 100 mL of hexane. The extract was then evaporated to dryness in a rotary evaporator, and dissolved in petroleum ether (1:3) for HPLC analysis.
HPLC analysis: Agilent series 1100 System equipped with automatic injector and a Microsorb-MV column C18 (250 mm×4.6 mm; 5μm particle size) was used.
The mobile phase (A) consisted of 2% triflouroacetic acid (2 ml TFA/1000 ml distilled water), and mobile phase (B) consisted methanol (HPLC grade) at a flow rate of 0.6 ml/min, with 30 μl injection volume. The gradient elution program used was: 100% A/0% B in 0 min; 10% A/90% B in 60 min. The fractions were detected at 280 nm.
Phenolic compounds were identified using standard substances and their UV characteristic and relative retention times.
Statistical analysis: The data was statistically analyzed by using the statistical package for social sciences (SPSS, version15.0, 2007, Chicago, IL). One way analysis of variance (ANOVA) test was performed to test difference between the samples followed by mean separation using Duncan’s Analysis. Significance was declared at p<0.05.
Gross composition
Table 1 depicts the gross composition of amurca. Carbohydrates, proteins, fats, moisture, ash and fiber were 0.74% ± 0.0.411, 0.7% ± 0.023, 49.43% ± 1.08, 47.33% ± 0.447, 0.89% ± 0.292 and 0.92% ± 0.292 respectively
| Composition | Percentage (%) ± SE |
|---|---|
| Carbohydrates | 0.74% ± 0.02 |
| Protein | 0.70% ± 0.02 |
| Fat | 49.43% ± 0.29 |
| Moisture | 47.33% ± 0.30 |
| Ash | 0.89% ± 0.05 |
| Fiber | 0.92% ± 0.03 |
| Total | 100.00 |
Table 1: Gross composition of amurca (%).
Gross composition of amurcawas not reported earlier except for nitrogen content which was found to be 0.6% [16]. The low quantity of protein and carbohydrates in amurcaand the high quantity of fat and moisture can be attributed to components of amurcawhich are vegetation water and olive tissue [1].
Total phenolic compounds
Table 2 shows total phenolic compounds of amurcaextracted from freshly pressed olive oil in comparison to total phenolic compounds of freshly pressed olive oil. Total phenolic compounds of amurca289.6 ± 0.402 mg GAE/100 g, which is 9.1 times higher than that of olive oil (31.62 ± 0.37 mg GAE/100 g) comes in agreement with Lozano- Sanchez and others who reported that extra virgin olive oil byproducts which settles over time at the bottom of the container is consider a natural source of phenolic compounds [17].
| Criteria | Amurca | Olive oil |
|---|---|---|
| Total phenolic compounds (mg GAE/100 g) | 289.6 ± 0.402 | 31.62 ± 0.37 |
| Antioxidant activity (mg vitamin E equivalent/100 g) | 22.3 ± 0.53 | 1.29 ± 0.057 |
| Free fatty acid value (Oleic acid%) | 1.62 ± 0.029 | 1.39 ± 0.034 |
| Peroxide value (meqO2/kg) | 1.78 ± 0.030 | 11.69 ± 0.0007 |
Table 2: Total phenolic compounds, antioxidant activity, free fatty acid and peroxide values of amurca samples extracted from olive oil after 12 months of storage in comparison with freshly pressed olive oil.
Antioxidant activity
Table 2 also depicts antioxidant activity of amurcain comparison to olive oil. Antioxidant activity of amurca(22.3 + 0.53 mg vitamin E equivalent/100 g) was 17 folds higher than that of freshly pressed olive oil (1.29 + 0.057 mg vitamin E equivalent /100g) (p<0.05). Frega et al. [17] also suggested that amurcadispersed in olive oil might have some antioxidant activity.
Free fatty acid and peroxide values
Table 2 depicts free fatty acid and peroxide values of amurcain comparison with those of freshly pressed olive oil. Olive oil standard according to the Jordanian Institution for Standards and Metrology free fatty acid value must be ≤ 3.3 as oleic acid percent and peroxide value must be ≤ 20 meqO2/kg olive oil.
Free fatty acid value for amurcawas 1.62 % ± 0.029 (p<0.05) which is statistically insignificant from the free fatty acid value of freshly pressed olive oil samples (1.39% + 0.034), which can be explained by stabilizing role of suspended amurcaagainst hydrolytic degradation of triglycerides [18,19]. On the contrary, it was reported freshly pressed olive oil that have a cloudy appearance had higher free fatty acid value and that filtration of cloudy olive oil decreases the rate of hydrolysis of triglycerides [20].
Furthermore, peroxide value of amurcasamples was 1.78 ± 0.030 meqO2/kg oil (p<0.05) which is significantly lower than that of freshly pressed olive oil sample 11.69 + 0.00, which can explained by the presence of high antioxidant activity in amurcathat inhibits the initiation stage of auto-oxidation of free fatty acids [21-23].
Lipid peroxidation
Figure 1 shows lipid peroxidation inhibition ratio of amurca extract in comparison with freshly pressed olive oil. Methanolic extract of amurcacaused significant LPO inhibition (96.1%), while freshly pressed olive oil caused 53.1% LPO inhibition. This can be attributed to high total phenolic compounds and antioxidant activity that inhibits MDA formation [24,25].
HPLC profile of phenolic compounds
Figure 2 shows the chromatogram of phenolic compounds of amurcaextract. The concentrations of the main phenolic compounds which were determined by the calibration curves obtained from their respective commercial standards are depicted in table 3. Oleuropen concentration was the highest amongst phenolic compounds in amurca extract (10.03 mg/g), isopropyl-5-methylphenol and sinapic acid were found to be 3.68 and 4.63 mg/g respectively, while the concentration of luteoline, gallic acid, kaempherol and 3-hydroxy phenol, were 2.7, 1.76, 1.49, and 4.94 mg/g amurcarespectively, which comes in agreement with findings of Montedoro et al. [25], Murkovic et al. [26], Servili et al. [27], Tuck and Hayball [28], Cardoso et al. [29] and Fu et al. [30] who reported that these phenolic compounds were the most prominent in olive oil or olive fruits.
| Phenolic Compounds | Sample Area | Stander Area | mg/g amurca |
|---|---|---|---|
| Oleuropen | 45.58152 | 2.00E+03 | 10.03 |
| Isopropyl-5-methyl phenol | 63.82699 | 6.07E+03 | 4.63 |
| Sinapic acid | 115.4224 | 1.38E+04 | 3.68 |
| Luteolin | 104.05997 | 1.70E+04 | 2.70 |
| Gallic acid | 100.08049 | 2.50E+04 | 1.76 |
| Kaempferol | 42.98399 | 1.27E+04 | 1.49 |
| 3-hydroxy phenol | 42.60352 | 37791.7 | 0.494 |
Table 3: Phenolic compounds of amurca samples.
We would like to thank the Deanship of Research at Jordan University of Science and Technology (JUST) for providing financial support for this project (9/2009).