Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2015) Volume 5, Issue 5
The objective of present study was to study the effect of biofield treatment on physical and thermal properties of gluten hydrolysate (GH) and ipomoea macroelements (IM). The study was performed in two groups (control and treated). The control group remained as untreated, and biofield treatment was given to treated group. The control and treated GH and IM were characterized by particle size analysis, surface area analysis, X-ray diffraction (XRD), Differential scanning calorimetry (DSC), and Thermogravimetric analysis (TGA). Particle size results of treated GH showed that d50 (average particle size) was decreased by 3.15% and d99 (size exhibited by 99% of powder particles) by 18.40% as compared to control GH sample. The treated IM also showed substantial reduction in average particle size d50 by 4.70% and d99 by 44.40% as compared to control sample. BET analysis showed significant increase in surface area of treated GH by 374.40 % as compared to control sample. However, the treated IM showed reduction in surface area by 14.30% as compared to control sample. XRD data suggested that both control and treated GH samples were amorphous in nature. Contrarily, the treated IM sample showed intense crystalline nature; though minimal decrease was observed in crystallinity as compared to control. DSC data showed increase in melting temperature of the treated GH as compared to control which can be correlated to alteration in kinetic energy of sample. Additionally, the DSC of treated IM also showed increase in melting temperature as compared to control IM. A significant increase in latent heat of fusion (ΔH) was observed in treated GH by 302.55% with respect to control. Similarly, the treated IM showed 24.87% increase in latent heat of fusion as compared to control. TGA data showed higher thermal decomposition temperature (Tmax) of treated GH as compared to control. However, treated IM showed that Tmax was decreased as compared to control sample. These results suggested that biofield treatment has substantially changed the physical and thermal properties of the treated organic products (GH and IM).
Keywords: Gluten hydrolysate; Ipomoea macroelements; Biofield treatment; Particle size; Surface area; XRD; DSC; TGA
GH: Gluten Hydrolysate; IM: Ipomoea Macroelements; XRD: X-ray Diffraction; DSC: Differential Scanning Calorimetry; TGA: Thermogravimetric Analysis
According to latest estimate of International Grist Council, the total output of corn in the world has reached 863 million tons in 2012 and 2013. Corn gluten meal is a main byproduct of corn wet milling and it contains almost 60% of protein [1,2]. Corn protein has less water solubility and lacks essential amino acids such as lysine and tryptophan, which reduces its applications in food industries and mainly it is used as animal feed. The major protein fractions of gluten meal are zein and glutelin representing 68 and 28% respectively of the total protein weight. The corn gluten hydrolysate (GH) was recently reported to have the anti-oxidant nature, which can be exploited for many applications [1]. Ipomoea macroelements (IM) has been used as a medium for cell, tissue and organ culture applications. Hence, in order to improve the functional properties of the GH and IM it should be modified in proper manner. Recently biofield treatment was used as an alternative approach to modify the physical, atomic and thermal characteristics of various metals [3-6].
Researchers have demonstrated that short lived electrical events or action potential exists in several types of animal cells such as neurons, muscle, and endocrine cells [7]. The law of mass-energy interconversion has existed in the literature for more than 300 years and the thought was initially described by Fritz [8], after that Einstein [9] derived the well-known equation E=mc2 for light and mass. However, the conversion of mass into energy is well established, but its inversion i.e., energy into mass has not yet proved scientifically. Furthermore, the energy can exists in several forms such as kinetic, potential, electrical, magnetic, and nuclear. Similarly, the human nervous system consist of the energy in the form of electrical signals [10,11]. Whenever these electrical signals fluctuate with time, the magnetic field generates as per the Ampere-Maxwell law, and cumulatively known as electromagnetic field. Hence, the electromagnetic field being generated from the human body is known as biofield energy [12].
Thus, human beings have the ability to harness the energy from environment/Universe and can transmit into any object (living or non-living) around the Globe. The object(s) always receive the energy and respond into a useful manner that is called biofield energy. This whole process is known as biofield treatment. Mr. Trivedi has transformed the properties of various living and non-living objects under controlled experiments using his unique biofield. The biofield treatment which is also known as The Trivedi Effect® has significantly changed the production and quality of various agricultural products [13-15]. Additionally, biofield treatment has shown excellent results in altering the antibiotic susceptibility, biochemical reactions pattern, as well as induced alterations in biotype characteristics of pathogenic microbes [16-18]. The biofield treatment had also caused increase in growth and anatomical characteristics of an herb Pogostemon cablin that is commonly used in perfumes, in incense/insect repellents, and alternative medicine [19]. Biofield treatment has substantially altered the medicinal, growth and anatomical properties of ashwagandha [20].
Based on important food applications of GH and microbiology uses of IM, the present study was undertaken to evaluate the impact of biofield treatment on physical and thermal properties of the organic products (GH and IM).
Experimental
The gluten hydrolysate (GH) and ipomoea macroelements (IM) powder were procured from HiMedia Laboratories Pvt. Ltd, India. Each sample was divided into two parts; one was kept as a control sample, while the other was subjected to Mr. Trivedi’s biofield treatment and coded as treated sample (T). The treatment groups (T) of both compounds were in sealed pack and handed over to Mr. Trivedi for biofield treatment under laboratory conditions. Mr. Trivedi provided the treatment through his energy transmission process to the treated group without touching the sample. The control and treated samples were subjected to characterization by particle size, surface area analysis, XRD, DSC and TGA.
Particle size analysis
The average particle size and particle size distribution of GH and IM were analyzed by using Sympetac Helos-BF laser particle size analyzer with a detection range of 0.1 micrometer to 875 micrometer. Average particle size d50 and d99 size exhibited by 99% of powder particles were computed from laser diffraction data table. The percentage changes in d50 and d99 values were calculated by the following formula:
Percentage change in d50 size=100 × (d50 treated - d50 control)/ d50 control
Percentage change in d99 size=100 × (d99 treated - d99 control)/ d99 control
Surface area analysis
The surface area of GH and IM powders were characterized by using surface area analyzer, SMART SORB 90 BET, which had a detection range of 0.1-100 m2/g. Percent changes in surface area were calculated using following equation:
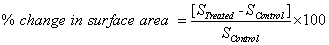
Where, S Control and S Treated are the surface area of control and treated samples respectively.
X-ray diffraction (XRD) study
XRD of GH and IM (control and treated) powders were analyzed by using Phillips Holland PW 1710 X-ray diffractometer system. The wavelength of the radiation was 1.54056 angstrom. The data was obtained in the form of 2θ versus intensity (au) chart.
Differential scanning calorimetry (DSC) study
The control and treated samples of GH and IM powder were used for DSC study. The samples were analyzed by using a Pyris-6 Perkin Elmer DSC on a heating rate of 10°C/min under air atmosphere.
Thermogravimetric analysis (TGA)
Thermal stability of the GH and IM powder of control and treated samples were analyzed using Mettler Toledo simultaneous TGA. The samples were heated from room temperature to 400ºC with a heating rate of 5ºC/min under air atmosphere
Particle size and surface area analysis
Particle size of the natural and organic materials plays an important role for its biological applications. The average particle size (d50) and d99 value of the treated and control organic products (GH and IM) are presented in Table 1. The d50 value of control GH was 8.56 μm, however, after bio-field treatment; it was decreased to 8.29 μm. The percentage change in average particle size d50 was calculated, and it was decreased by 3.15% in the treated GH sample as compared to control. Whereas the d99 value in control GH was 54.29 μm and after treatment it was decreased to 44.28 μm. The percentage decrease in d99 value was 18.4% in treated GH with respect to control. Similarly, the d50 and d99 values of the treated (d50; 54.21 μm and d99; 202 μm) IM was decreased as compared to control sample (d50; 56.86 μm and d99; 363.4 μm). The percentage decrease in d50 and d99 of IM sample was 4.7% and 44.4%, respectively. It is presumed that biofield energy may deeply absorbed by the IM and GH particles that might have induced enormous stress on internal boundaries of particles, which may cause significant reduction in d50 and d99 value. Additionally energy milling induced through biofield treatment might also cause reduction in particle size of treated GH and IM with respect to control.
| Material | Average particle size d50 (μm) | Particle size d99 (μm) | % change | |||
|---|---|---|---|---|---|---|
| Control | Treated | Control | Treated | d50 | d99 | |
| Gluten hydrolysate | 8.56 | 8.29 | 54.29 | 44.28 | -3.2 | -18 |
| Ipomoea macroelements | 56.86 | 54.21 | 363.4 | 202 | -4.7 | -44 |
Table 1: Particle size results (d50 and d99) of gluten hydrolysate and ipomoea macroelements.
Mechanical treatment has been used as useful method for reduction of particle size and several milling devices have been explored for this purpose. Few methods such as freeze milling method have been used for cellulose homogenization, and wet milling is often used in food industry [21-24]. Nonetheless, all these mechanical methods might deteriorate the physical properties of the polymers and also these methods are time consuming. Therefore, in present work the biofield treatment was used to effectively reduce the particle size of organic products (GH and IM). The biofield treated samples showed substantial reduction in particle size that can be used for biomaterial and food applications.
The surface area of the GH and IM were analyzed by BET and results are listed in Table 2. The surface area of treated GH (1.38 m2/g) was increased as compared to control sample (0.29 m2/g). However, the treated IM (1.00 m2/g) showed decrease in surface area as compared to control sample (1.16 m2/g). The percentage changes in surface area of the samples were computed from the BET results (Table 2). The treated GH showed significant increase in surface area by 374.4% as compared to control. Since the surface area and particle size changes are usually opposite to each other i.e. smaller the particles size, larger the surface area and vice versa [25,26]. Hence, decrease in particle size caused to increase in surface area of treated GH and this may increase its solubility. The enhanced solubility of GH might improve its food applications. However, IM showed a decrease in percentage surface area by 14.3%. It is assumed that biofield treatment may cause pore or irregular surface formation on the treated IM particle that may led to decrease in surface area.
| Material | Surface area (m2/g) | % change in surface Area | |
|---|---|---|---|
| Control | Treated | ||
| Gluten hydrolysate | 0.29 | 1.38 | 374.4 |
| Ipomoea macroelements | 1.16 | 1 | -14.3 |
Table 2: Surface area analysis of control and treated samples (Gluten hydrolysate and Ipomoea macroelements).
X-ray diffraction studies
X-ray diffract gram of control and treated GH are shown in Figure 1. XRD diffractogram of control GH showed a broad diffraction peak at 2θ equals to 22°, which showed the amorphous character of the sample. However, after treatment this broad halo peak was observed (Figure 1) at lower diffraction angle (2θ=20°). This was may be associated with increased amorphousity in the treated sample. It was previously reported that XRD analysis of corn samples at low temperature normally shows broader and flatter peaks [27]. Moreover, the presence of water also affects the intensity of the XRD peaks in corn based polymers. It was postulated that probably presence of bound water and biofield treatment reduced the crystallization in treated GH sample. In a similar research article published by Hibi et al. [28] who reported that high pressure treatment destroys the crystallinity of the normal corn or maize samples [28].
Contrarily, the IM showed (Figure 2) a well-defined crystalline nature with intense diffraction peaks at 2θ equals to 23.55°, 28.18°, 28.34°, and 33.81°. These peaks clearly showed the crystalline nature of the IM powder. The crystalline nature was due to regular or periodic arrangement of molecules in IM crystals. The biofield treated IM powder also exhibited (Figure 2) crystalline nature. The diffractogram showed intense crystalline peaks at 2θ equals to 23.47°, 28.15°, and 33.00°. The intensity of the crystalline peaks was minimally decreased in treated IM which may be due to biofield treatment, that caused disturbance in regular pattern of the sample and hence, alteration in crystallinity with respect to control.
DSC study
The DSC thermograms of control and treated GH are presented in Figure 3. The thermogram of control GH showed (Figure 3) an endothermic peak at around 130.27°C that was may be associated with its melting temperature. The lower melting temperature of the control sample was due to amorphous nature and this was also supported by XRD data of the control GH. However, the DSC thermogram of treated GH showed an increase in endothermic inflexion at 163°C which was may be due to rigid nature of the GH macromolecular chain. The increase in melting temperature of treated GH (Figure 3) was may be due to biofield which induced long range order in GH atoms and this led to change in thermal nature. It was previously reported that melting temperature of materials depends on kinetic energy of the sample [29]. Hence, it is presumed that biofield may alter the kinetic energy of molecules of polymer that probably led to increase in melting temperature.
The control IM sample showed (Figure 4) a sharp endothermic peak at 131°C that was due to bound water in the sample. The control sample showed another endothermic transition peak at 273°C which was associated with the melting temperature. The DSC thermogram of treated sample exhibited (Figure 4) sharp endothermic transition at 132.38°C that was due to elimination of loosely bound water in the sample. Another endothermic peak was clearly evident at around 277°C that was associated with the melting temperature of the IM. This showed the clear elevation in melting temperature of the treated IM as compared to control. It is assumed that biofield treatment may cause changes in kinetic and vibrational energy of treated IM that led to increase in melting temperature as compared to control.
In a solid substantial amount of intermolecular forces exist between the atomic bonds to hold the atoms at their respective positions. Latent heat of fusion (ΔH) is the energy required to overcome this interaction force in order to change it from solid phase to liquid. Thus the energy supplied during this phase change i.e. ΔH is stored as potential energy in atoms [29]. The ΔH was calculated for control and treated organic products and depicted in Table 3. The control GH showed a ΔH of 34.78 J/g and it was increased to 140.01 J/g in treated GH. However, the treated IM showed ΔH of 23.08 and it was increased to 28.82 J/g in treated IM sample. After biofield treatment significant change in ΔH was observed in treated GH and IM with respect to control. The treated GH showed increase in ΔH by 302.56% as compared to control. Likewise in treated IM it was increased by 24.87% as compared to control. It is assumed that bio-field treatment may induce changes in potential energy of the treated organic products that led to increase in ΔH.
| Sample | Control (∆H J/g) | Treated (∆H J/g) | % Change in ∆H |
|---|---|---|---|
| Gluten hydrolysate | 34.78 | 140.01 | 302.55 |
| Ipomoea macroelements | 23.08 | 28.82 | 24.87 |
Table 3: Latent heat of fusion (ΔH) of control and treated gluten hydrolysate and ipomoea macroelements.
Thermal stability (TGA)
TGA is commonly used to investigate the thermal stability of the samples. Figure 5 shows the TGA thermogram of control and treated GH. The TGA thermogram of control GH showed three step thermal degradation pattern (Figure 5). The sample showed elimination of bound water at around 72-105°C. During this thermal event the sample lost 16.83% of its weight. The second thermal degradation event was observed at 220-255°C and sample lost 12.54% of its original weight.
Another thermal decomposition event was observed at 260-290°C and the sample lost 12.91% of its weight. This thermal event may be related to degradation of the polymer chain. The treated GH demonstrated two step thermal degradation pattern. The treated sample showed (Figure 5) first thermal degradation step at around 135-175°C and during this process sample lost 7.76% of its weight. The second thermal degradation was observed at 228-370°C. During this step treated sample lost 26.09% of its weight. DTG thermogram of control GH showed Tmax at 269°C.
However, the treated GH showed Tmax at 157°C. This showed the reduction in thermal stability of treated GH with respect to control.
TGA thermogram of control and treated IM are shown in Figure 6. The thermogram of control IM demonstrated two-step thermal decomposition step. The thermal degradation was observed at around 185-246°C and during this process sample lost 10.84% of its weight. DTG showed the Tmax at 218°C. Another weight loss step was observed at 260-290°C, which was may be due to degradation of the sample.
During this step sample lost 2.32% of its weight. Contrarily the biofield treated sample showed (Figure 6) one step thermal degradation at 82-156ºC and the sample lost 5.47% of its total weight. Based on the evaluation of DTG thermogram of treated IM the Tmax was observed at 131°C. DTG results showed higher Tmax in control sample as compared to treated sample. Hence, we presume that treated sample was less thermally stable than control IM. It is assumed that biofield treatment may cause disturbance in symmetrical or regular pattern in the treated IM atoms that led to maximum decomposition at low temperature and hence, reduction in thermal stability.
This research work showed the impact of biofield treatment on physical and thermal properties of GH and IM. Particle size analysis of treated GH and IM showed substantial reduction in particle size as compared to control, which may be due to energy milling induced by biofield treatment. The particle size analysis result of treated GH and IM showed substantial reduction in particle size with respect to control. BET analysis of treated GH showed considerable increase in surface area which could improve its food applications. However, treated IM showed reduction in surface area with respect to control. XRD of control and treated GH showed amorphous nature. Additionally, the treated IM showed alteration in crystalline nature with respect to control. DSC analysis showed increase in melting temperature of treated samples (GH and IM) as compared to control. Latent heat of fusion was substantially enhanced in treated samples (GH and IM) with respect to control. TGA analysis of treated GH showed reduction in thermal stability with respect to control. Likewise, TGA of treated IM also showed reduction in thermal stability as compared to control. Overall, the result showed that biofield treatment has substantially changed the physical and thermal properties of treated GH and IM with respect to control. Based on the results, it is presumed that biofield treated GH and IM could be used as matrix for food applications and as medium for microbial cell culture, respectively.
We thank Dr. Cheng Dong of NLSC, Institute of Physics, and Chinese academy of Sciences for permitting us to use Powder X software for analyzing XRD results. The authors would like to also thank Trivedi Science, Trivedi Master Wellness and Trivedi Testimonials for their support during the work.