Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Research Article - (2023)Volume 8, Issue 1
The aim of this study was to obtain valuable information about the effect of ultrasonic irradiation with a frequency of 20 kHz while discontinuing pulsation (10 s off, 5 s on) on the inactivation capability of Staphylococcus aureus ATCC 25923, in physiologic water samples.
Ultrasonic irradiation of bacterial samples with different populations of 108, 1010 and 1012 colony-forming units/mL by varying the volumes of the bacterial suspension (15 mL, 30 mL and 45 mL) was performed at a constant frequency with different amplitude levels 60%, 80% and 100%, various treatment times and temperatures 20, 30 and 50°C ± 2°C. The linear section of a plot showing a survival ratio logarithm vs. sonication time was used to determine the rate constant for ultrasonic inactivation. In spite of the fact that ultrasonic therapy resulted in a large mortality rate at 80% and 100%, contrary to predictions, a rise in S. aureus populations was seen at 60%.
The findings of this study suggest that ultrasound irradiation is a suitable method for the elimination of the main pathogen, such as S. aureus, at high amplitude 80% at temperatures that reach 50°C, given the widespread use of ultrasound for the sterilization of tools and equipment used in hospitals.
Ultrasound; Staphylococcus aureus; Inactivation; Sterile physiological water
The key issue is Foodborne Diseases (FBD). Unhealthy food has a significant worldwide influence on health, trade, and wealth, but its precise effects on wellbeing and overall economic loss are unclear [1].
The true number of FBD is difficult to ascertain due to systematic underreporting and challenges in attributing illnesses with multiple modes of transmission to food consumption. Despite this, significant progress has been made in detecting the FBD burden, and reliable data from many developing nations shows that a sizeable fraction of the population-between 12% and 33%-is afflicted annually. Staphylococcus aureus has a significant involvement in several of these estimations of multipathogen diseases [2,3].
Staphylococcus aureus (S. aureus) is a gram-positive, catalasepositive, facultative aero-anaerobic, nonsporulating, nonmotile bacterium that manifests as a cluster shell (bunches of grapes). The S. aureus species is a common germ that lives in the environment and is commensal to humans. It may be found in the air, dust, sewage, water, environmental surfaces, people, and animals [4,5]. It is a halophilic bacteria that can thrive in a variety of temperatures and pH levels and tolerates high salt concentrations [6].
In some environments or situations, S. aureus is an opportunistic pathogen. Its pathogenicity is linked to a number of distinct secretions, including toxins (endotoxins) and enzymes (coagulase) [4]. According to research by Bhatia A, et al. [4], at least 105 S. aureus cells are required in one gram of food in order for the organism to secrete enough enterotoxin to be harmful.
Given that it satisfies the food and drug administration's criteria for a 5-log decrease of microbial cells in fruit juices, ultrasound is regarded as an effective procedure [7]. Also, the "non-thermal" nature of it reduces the negative effects of heat on the nutritional and organoleptic properties of the food product [8,9]. In addition to all of these characteristics, it is regarded as a "green" technology since it uses healthy energy and, in certain cases, no extra chemicals. In comparison to the physical and chemical processes used in traditional extraction, processing, or preservation approaches, ultrasound exploits phenomena that are fundamentally different [10]. The main objective of this study was to evaluate the inactivation of S. aureus in sterile physiological water at different temperatures, amplitude and microbial load.
Bacterial strain and inoculum preparation
To create a final working stock bacterial culture, a loop of a bacterial colony from Staphylococcus aureus ATCC 29213 was added to 5 ml of Tryptic Soy Broth (TSB; Biokar Diagnostics, France). This was then incubated at 37°C overnight. The final bacterial suspension was made by adding 4 ml of the working culture to 100 ml of fresh TSB and putting it in a shaking bath at 37°C. For this last stage, the time of incubation was changed so that the bacteria culture was in the exponential phase (also called the "log phase").
Using a spectrophotometer at a wavelength of 600 nm, the change in the optical density of the culture solution over time was measured to record the microorganism growth curve (Novaspec II Visible Spectrophotometer). Hettich Rotina 380 R centrifuge from Germany was used to collect cells in the exponential phase. Cells were centrifuged at 10,000 g for 10 minutes at 4°C, washed three times, and then resuspended in buffered peptone water (Biokar diagnostics, France). Bacterial suspensions with an initial concentration of 1012 CFU/mL were made using optical density measurements and plate counting [11].
Treatment of Staphylococcus aureus ATCC 29213
The ultrasound homogenizer (VCX 1500, Sonics and Materials, Inc. Newtown, Connecticut, USA) was used to ultrasonicate 15 ml of distillate water that contained 1012 CFU/ml of S. aureus at a constant frequency of 20 kHz while discontinuing pulsation (10 s off, 5 s on). To highlight the effect of amplitude, temperature, charge and volume of the treatment bacterial suspension several experiments were performed.
The effect of different amplitudes: Different amplitude levels 60%, 80% and 100% were tested on 15 ml of a cell suspension (1012 CFU/ml) for 20 minutes, the temperature of the suspension being treated was maintained at 30°C ± 2°C. To keep a steady temperature, the water bath is connected to the ultrasonic processor (Figure 1).
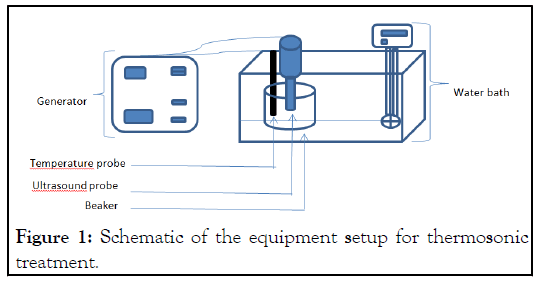
Figure 1: Schematic of the equipment setup for thermosonic treatment.
Three replicates of all these experiments were performed.
Influence of bacterial load, bacterial suspension volume: 15 ml of a cell suspension at the different initial load (108 UFC/mL, 1010 UFC/mL and 1012 UFC/mL) were tested at 80% for 20 minutes, the temperature of the suspension being treated was maintained at 30°C ± 2°C. A water bath and an ultrasonic processor are used to keep the temperature steady. The nature of the impulses was the same during all the manipulations (5 s on/10 s off).
The same manipulation was performed by varying only the volumes of the bacterial suspension (15 mL, 30 mL and 45 mL).
Three replicates of all these experiments were performed.
Influence of the temperature of the bacterial suspension: 15 ml of a bacterial suspension of 1012 CFU/ml were treated with ultrasound using an ultrasound generator (VCX 1500, Sonics and Materials, Inc. Newtown, Connecticut, USA) at 1500 W and a constant frequency of 20 kHz and amplitude equal 80% (96 μm) for 20 minutes. The temperature of the suspension being treated was varied from 20, 30 and 50°C ± 2°C using a cooling device with pulse durations of 5 s and 10 s off. The suspension to be treated was placed in a 50 ml jacket and ultrasonically treated by immersing a 10 mm diameter probe (operational immersion depth of 1.5 cm). Aliquots of 100 μL of bacterial suspension were distributed in sterilized 900 μL micro tubes. After ultrasound treatment, microbial survival was examined. As a control, an untreated inoculation sample was employed.
Enumeration of bacteria
To determine the S. aureus microbial load, serial dilutions were carried out in buffered peptone water with distilled water containing S. aureus collected before and after treatments. Spread plating the diluted samples onto the S. aureus-selective enumeration medium Mannitol Salt Agar (MSA, Biokar Diagnostics, France) allowed researchers to identify any stressinduced sub lethal cells present in the water samples. The plates were incubated for 24 hours at 37°C. All microbiological examinations were carried out in triplicate for each experiment. The agar plate culture colony count technique is used to calculate the number of Colony-Forming Units (CFU) per milliliter (CFU/ml) of bacterial solution.
Statistical analysis
Statistical analysis was performed on the data obtained from three independent experiments. ANOVA was used to determine significance levels at the 5% probability level using Statistica 1.5 software.
Growth curve
The systematic expansion of all bacterial components is referred to as bacterial growth. A stationary period, an exponential phase, a delay phase, and a decline phase make up the growth curve [12,13]. By observing the variations in absorbance over time, the S. aureus growth curve was captured (Figure 2). As can be seen, after 3 hours of incubation, the S. aureus suspension entered the mid-exponential phase. The growth rate reaches its maximum (μ=max), during this phase, and all of the cells in the cell mass are still alive (zero mortality) [13]. After incubation for 7 hours, the cells reached the stationary phase, when the population size is constant and the growth rate is zero (μ=0). The findings of this research are consistent with those of Li, et al. [3] report on Staphylococcus epidermidis.
Figure 2: Growth curve of Staphylococcus aureus.
It takes time for the bacteria to produce the enzymes necessary for the new substrate during the latency phase, which depends on the age of the bacteria and the make-up of the growth medium. The bacteria exhibit no latency period when transplanted to the same medium [12]. We may interpret S. aureus' actions in this experiment based on this definition. The use of the same culture medium throughout the revivification and realization of the growth curve accounts for the lack of the latency period.
Belay, et al. [14], found that the culture conditions affect the bacteria's generation time after examining the development of S. aureus and the formation of Staphylococcal Enterotoxin A (SEA) under aerobic and anaerobic environments. They discovered that it took 80 minutes and 35 minutes, respectively, for anaerobic and aerobic conditions to generate to mid-log phase. They discovered that bacterial growth affects the production of SEA, but that SEA was only detected after 120 minutes of incubation in both cases [15].
Effect of amplitude
In our study, the temperature was set at 30°C. The suspension treated at 100% and 80% amplitude for 20 minutes showed inactivation of Staphylococcus aureus from an initial load of approximately ≈1012 UFC/ml up to 4 log and 2 log respectively, a declumping effect of 2 log was seen when the suspension was treated at 60% amplitude; therefore, higher amplitudes and longer treatment times were more effective for microbial inactivation (Table 1).
| Amplitude | Charge | N/N0 | log |
|---|---|---|---|
| 60% | 5.00E+14 | 500 | 2,69,897 |
| 80% | 6,7E+10 | 0,067 | -1,17,39,252 |
| 100% | 5,00E+08 | 5,00E-04 | -3,30,103 |
Table 1: Effect of amplitude on S. aureus reduction rate.
Tao Y, et al. [16], Kernou ON, et al. [17,18], estimated that it is possible that ultrasound applied to microbial suspensions disperses aggregates of microorganisms, disrupts cells, and alters cellular activity from the outside to the inside of structures.
These effects result from the combined physical and chemical mechanisms that occur during cavitation bubble collapse, free radical formation (e.g., OH"), and hydrogen peroxide production [19,20].
As more energy is emitted at higher intensities, germs are typically more inactivated the higher the intensity and amplitude of the ultrasound applied. It was discovered that increasing the ultrasonic power improved the inactivation rate of Mycobacteriumi sp. 6PY1 [21]. Several outbreaks of E. coli that have resulted in fatalities have received extensive media attention [22]. E. coli XL1-Blue's susceptibility to ultrasonic inactivation was examined using a horn-type sonicator that used the squeeze-film effect at 27.5 kHz with a high power intensity [23]. The findings demonstrated that the rate of bacterial inactivation rose as the vibrating face's amplitude grew, reaching more than 99% in 180 seconds at an amplitude of 3 m (p-p) and 2 mm squeezing film [23]. Moreover, E. coli samples ATCC 25922 and NCTC 12900 were studied using ultrasound at various amplitude levels (0.4 μm, 7.5 μm, and 37.5 μm) [24].
When ultrasonic treatment was used for 15 minutes at a wavelength of 37.5 m or less and an amplitude of 7.5 m, it was found that the amount of E. coli was cut by more than 5 logs [24]. When S. cerevisiae was sonicated at a frequency of 20 kHz, it was also found that the rate of disruption and protein release increased when the power went from 120 to 600 W [25].
The number of total microorganisms was considerably reduced at higher intensities than at lower intensities when a batch of aerobic mesophilic bacteria was ultrasonically treated in date syrup at both 10% and 25% of total power (the specific power or intensity employed was not given) [26]. Nevertheless, the inactivation rate of Mycobacteriumi sp. 6PY1 was observed to achieve a constant level at low frequency when the power reached a particular level. And to explain this, it was proposed that the number of cavitation bubbles must match the number of bacteria [21,27].
Effect of load and volume of the bacterial suspension
Studies on the relationship between the effectiveness of ultrasonic inactivation and the initial bacterial count have been scarce. Our results indicated that the kill rate was independent of the initial bacterial count (Table 2).
| Charge N | Charge N0 | N/N0 | log |
|---|---|---|---|
| 1,00E+08 | 1,97E+07 | 1,97E-05 | -47,06,26,924 |
| 1,00E+10 | 5,00E+08 | 5,00E-04 | -3,30,103 |
| 1,00E+12 | 2,54E+07 | 2,54E-05 | -45,94,59,672 |
Table 2: Effect of loading on bacterial reduction rate of S. aureus.
Some studies found that ultrasonic damage had nothing to do with how many bacteria were there to begin with, but that damage to the cells of different types of bacteria was caused in different ways [3].
The bulk of papers claimed that low beginning concentrations of microorganisms are better for the ultrasonic inactivation of bacteria than large initial concentrations. A concentration of 4 × 106 CFU/ml was found to inactivate 99.9% of E. coli K12 in 3 minutes, but a concentration of 2 × 109 CFU/ml required 4 minutes to have the same effect (20 kHz, 12.57 W/cm3) [28]. Low starting cell concentrations were more susceptible to ultrasound than high initial cell concentrations (approximately 1.8-log decrease for 102 cell/ml vs. about 0.3-log reduction for 105 cell/ml) when S. cerevisiae A50 was ultrasonically treated by a horn-type sonicator at 27.5 kHz [29]. It was also noted that the initial concentrations had no appreciable impact on the inactivation process. For instance, ultrasound at 20 kHz and 612 kHz for 70 minutes at protein concentrations of 2.15 × 10-3 to 1.4 × 10-2 mg/L inactivated suspensions of Mycobacteriumi sp. 6PY1. The concentration in this investigation was expressed as the quantity of protein per liter. The results shown that a removal of around 93% and 35.5% at 20 kHz and 612 kHz, respectively, was accomplished regardless of concentration [21]. Due to the increase in viscosity and the clumping of the microorganisms, it would seem logical that a greater quantity of bacteria would result in a slower rate of inactivation. The link between the starting number and the degree of bacterial inactivation, however, may also be influenced by other factors, such as the properties of the bacteria and ultrasonic energy.
From a few milliliters to more than 1l, the volume of bacteria employed in various investigations varies greatly [30,31]. The volumes used depend on the ultrasonic apparatus and the experiments' goals. Typically, when the same ultrasonic setup and settings are employed, the inactivation rate decreases with the rising volume. This is because a small volume of working suspensions has a higher real ultrasonic energy supplied per milliliter of media (i.e., W/ml) than a big one [32]. For instance, it was discovered that when sonicated at 20 kHz for 15 minutes, about 70% and 30% of B. subtilis were inactivated in quantities of 100 ml and 150 ml, respectively, whereas essentially no bacteria were inactivated in a volume of 200 ml [33]. In another investigation, when the amount of whole egg liquid was raised from 12.5 ml to 25 ml, the efficiency of ultrasonic treatment for Salmonella enteritidis was reduced; a log reduction of 2.30 and 1.62, respectively, was recorded [33]. When treated in an ultrasonic bath at 42 kHz with a power of 70 W for 30 minutes, in volumes of 200 ml, 400 ml, and 600 ml, respectively, the clearance percentage of E. coli was 90%, 86%, and 85% [34]. These findings strongly showed that microbial inactivation is volume dependent, requiring more power to achieve the same efficacy (Table 3).
| Charge N | Charge N0 | N/N0 | log |
|---|---|---|---|
| 1,00E+08 | 1,97E+07 | 1,97E-05 | -5,14,46,828 |
| 1,00E+10 | 5,00E+08 | 5,00E-04 | -5,28,12,216 |
| 1,00E+12 | 2,54E+07 | 2,54E-05 | -5,15,07,853 |
Table 3: Effect of volume on bacterial reduction rate.
Effect of temperature
The ultrasonic waves create large cavitation bubbles that collapse and create powerful jets exerting strong shear forces in the liquid [35]. The bacterial population rate 1012 CFU/ml in contaminated water decreased, using a frequency of 20 kHz at different temperatures (20, 30 and 50°C ± 2°C) with a time period (5, 10, 15 and 20 minutes).
The Figure 3 show that after 5 minutes of sonication, the number of colonies had been reduced to 4.81, 5.01 log UFC/ml at a temperature of 20°C and 30°C respectively, also by extending the sonication time to 20 and 40 minutes at the same frequency, the number of colonies decreased to 5.96, 5.99 log CFU/ml, and to 7.07, 7.36 log CFU/ml at 20°C and at 30°C respectively.
Figure 3: Inactivation of Staphylococcus aureus at 20 kHz, 80% amplitude. Note: 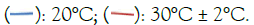
For S. aureus, the process was coordinated with membrane deterioration and intracellular esterase deactivation. Consequently, ultrasound may also inactivate the cytoplasmic membrane and internal cell structure [36].
Experimental results show that the number of S. aureus colonies decreases with increasing sonication treatment time. As the period increased, the bacterial population (i.e., CFU) decreased. These results can be interpreted by the ultrasonic wave shocks hitting the microbial cell walls, the increase in bubble implosion give a high mechanical effect, resulting in the destruction of bacteria. The temperature increased slightly in each sample with increasing treatment time. When the temperature is set between 20 and 30°C, it is called ultrasonic killing, because the temperature range for growth of S. aureus is between 7 and 48°C, with an optimum of 37°C. For bacteria, however, it is clear that a decrease in the number of colonies is observed.
Longer sonication times have more effect on the decreasing bacterial population compared to short treatment times. In addition, during the ultrasonic treatment, the microorganisms are also subjected to mild temperatures (50°C) (Figures 4 and 5), which increase the weakening of the bacterial membrane and possibly additional lysis attributed to cavitation [37].
Figure 4: Inactivation of S. aureus at 20 kHz, 80% amplitude. Note: 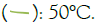 : 50°C.
: 50°C.
Figure 5: Inactivation of Staphylococcus aureus at 20 kHz, 80%amplitude. Note: 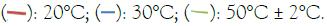
Therefore, the combined effects of ultrasound treatment and temperature may explain these results. Similar observations were reported by Herceg, et al. [38], who revealed that the magnitude, duration and temperature of an ultrasound treatment of milk significantly affected the inactivation of E. coli.
The unique specifications of sonication used in this study should be effective on the inactivation of gram positive bacteria, especially if sonication is applied for prolonged periods. In addition to increasing the population of S. aureus from its original value, the application also increases it from a control population that is not subjected to ultrasonic treatment. This method may be inefficient in getting rid of the main hospital bacteria S. aureus because to the extensive use of ultrasound for sterilizing tools and equipment in hospitals. To investigate the effects of ultrasonic irradiation on microbial decomposition at various specific energy inputs and longer sonication periods, however, in-depth future study is necessary.
[Crossref] [Google scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Akila A, Nadir A, Nouara OK, Amine B, Kamelia K, Khodir M(2023) Ultrasound Treatment Effects on Staphylococcus aureus Cell. J Food Microbial Saf Hyg. 8:188
Received: 08-Mar-2023, Manuscript No. JFMSH-23-22071; Editor assigned: 10-Mar-2023, Pre QC No. JFMSH-23-22071 (PQ); Reviewed: 24-Mar-2023, QC No. JFMSH-23-22071; Revised: 31-Mar-2023, Manuscript No. JFMSH-23-22071 (R); Published: 07-Apr-2023 , DOI: 10.35248/2476-2059.23.08.188
Copyright: ©2023 Akila A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited