Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Paper - (2020)Volume 10, Issue 5
Antibiotic treatments have been associated with the alteration of the microbiota and the killing or growth suppression of antibiotic-sensitive bacteria, with as result an overgrowth of opportunistic and resistant bacteria. This intestinal disbalance can be linked to clinical symptoms such as antibiotic-associated diarrhea (AAD) that can occur up to 8 weeks after antibiotic treatment.
Saccharomyces boulardii; Probiotics; antibiotics
In literature, the incidence of AAD is described to be up to 35%, mostly depending on the type of antibiotics used. A higher occurrence of AAD is seen with broad spectrum antibiotics affecting anaerobic bacteria [1-5]. One of the possible methods to restore the gut microbiome is the use of probiotics [2,6,7]. In this way, the microbiota can be made more colonization resistant, increasing the ability of the microbiota to resist overgrowth by pathogenic organisms [2,6,7]. Possible probiotic mechanisms are receptor and nutrient competition, inhibition of epithelial and mucosal adherence, induction of lower colonic pH favoring the growth of non-pathogenic species, the stimulation of immunity and the production of antimicrobial substances [6-8]. Additionally to the favorable effects, probiotics are easy to take, have a low cost and are safe to use [5,7]. This trial investigated the possible synergistic effect of a specific probiotic preparation containing Lactobacillus acidophilus NCFM, Bifidobacterium lactis Bi-07, Bi- 04, Lacticaseibacillus paracasei Lpc-37 and Saccharomyces boulardii, compared to Saccharomyces boulardii alone and placebo.
Patient selection
Patients receiving the antibiotic amoxicillin-clavulanate initiated in a hospital intensive care unit were screened for inclusion in the trial. Inclusion criteria were: treatment with amoxicillin-clavulanate and no change in current dietary habits. Exclusion criteria were: younger than 18 years, pregnancy, breastfeeding, average number of formed bowel movements more than 3 per day or less than 3 per week, participation in a clinical research trial within 30 days prior to randomisation, regular use of pro- and/or prebiotics, unstable medical condition, history of chronic gastrointestinal disorders including irritable bowel syndrome, colitis, Crohn’s disease, allergy or sensitivity to test product ingredients or antibiotics, patients receiving dialysis, deglutition abnormalities prohibiting or preventing a normal oral intake and a treatment with other antibiotics at the time of randomisation. 132 patients were equally randomized in three treatment groups: Probiotics, Saccharomyces and Placebo.
Study products
The probiotics study product contains Saccharomyces boulardii 6 × 109 CFU/dose, Lactobacillus acidophilus NCFM 2 × 109 CFU/ dose, Bifidobacterium animalis subsp. lactis Bi-07 2 × 109 CFU/ dose, Bifidobacterium animalis subsp. lactis Bi-04 2 × 109 CFU/dose, Lacticaseibacillus paracasei Lpc-37 2 × 109 CFU/dose (Probactiol duo®, Metagenics Europe). The Saccharomyces study product contains: Saccharomyces boulardii 6 × 109 CFU/dose while the placebo study product only contains excipients and has a similar look as the other two study products. Patients took 1 capsule 2 × day from the start of antibiotic treatment till 2 weeks after the antibiotic treatment.
Stool analysis
A stool sample was collected in a sterile container at the time of inclusion in the trial (Visit 1), at the end of the antibiotic treatment: (Visit 2) and at the end of the study treatment, one to three months after the discharge from the hospital (Visit 3). Samples were inoculated within 2 hours of reception with a 10 μL-loop on three selective media: chrom ID ESBL (bioMérieux, France), chromID VRE (bioMérieux, France,) and chromID Carba SMART agar (bioMérieux, France) and incubated aerobically at 37°C for 24 h which was prolonged to 48 h if no growth was observed at 24h. Most of the selective media are loaded with broad-spectrum third generation cephalosporins (C3). The cultured organisms were identified by matrix assisted laser desorption ionization timeof- flight mass spectrometry (MALDI-TOF MS; Bruker Biotyper, Bruker Daltonics, Germany).
Questionnaires
At visits 1, 2 and 3, patients were asked to complete the Gastrointestinal Symptoms Rating Score (GSRS). Additionally, patients were asked to complete a daily bowel habit diary including questions on the daily stool frequency and requesting an indication if there were any difficulties to start or stop the defecation. During the hospitalization, episodes of diarrhea were marked in the patient’s medical file.
Ethical committee
Ethics approval was obtained from the Comiteé Hospitalo- Facultaire Universitaire de Liège 707. All patients participating in the trial signed the informed consent form. The study was conducted according to ICH GCP guidelines and the declaration of Helsinki.
A total of 121 patients were included into the analysis of the three treatment arms: 40 in the placebo group, 42 in the probiotics group and 39 in the Saccharomyces group. Drop-out was due to non- compliance (<80% treatment compliance) and decease due to severe illness during the hospitalisation in the intensive care unit. Patient characteristics were similar in all groups with a mean age of 77.8 years. For 50 patients another antibiotic was added to the therapy or a switch to another antibiotic occurred during the study.
The MALDI-TOF MS analysis of the cultured stool samples showed a higher and more diverse colonisation with C3 resistant bacteria in all 3 groups after the antibiotic treatment indicating the microbial disturbance (Figure 1). This colonisation stabilizes at the end of the treatment in the placebo and Saccharomyces group while a remarkable decline in colonisation with C3 resistant bacteria is seen in the probiotics group, showing a synergistic effect of the probiotic strains in combination with Saccharomyces boulardii.
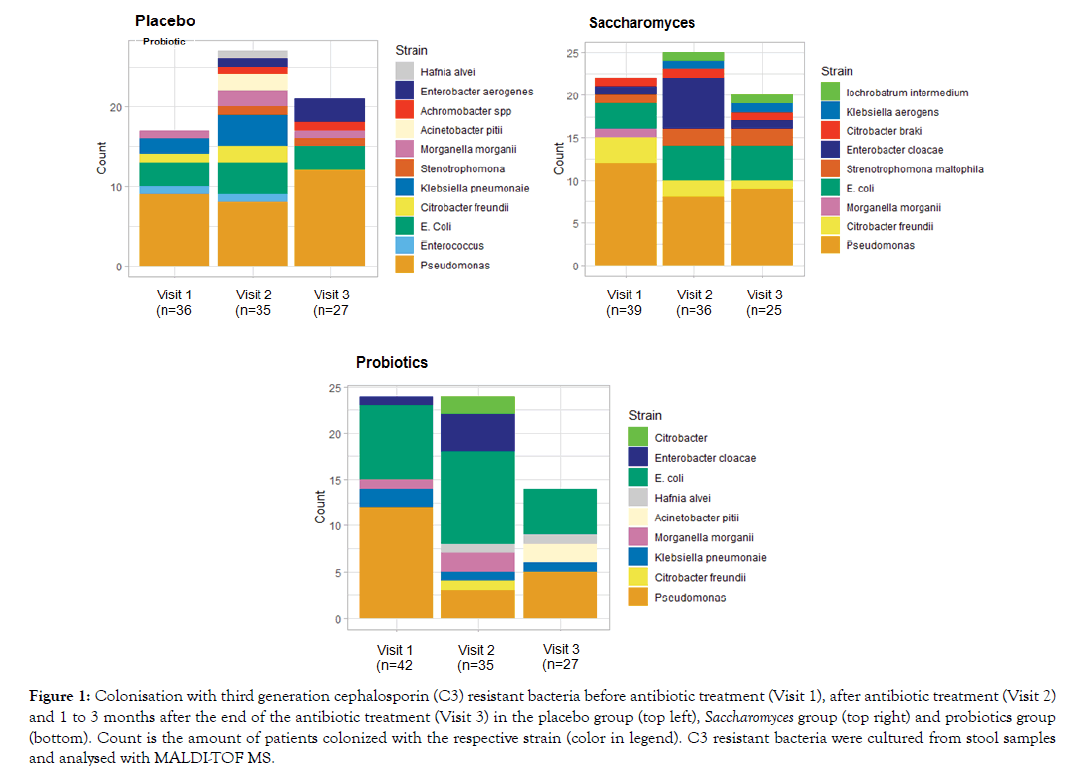
Figure 1: Colonisation with third generation cephalosporin (C3) resistant bacteria before antibiotic treatment (Visit 1), after antibiotic treatment (Visit 2) and 1 to 3 months after the end of the antibiotic treatment (Visit 3) in the placebo group (top left), Saccharomyces group (top right) and probiotics group (bottom). Count is the amount of patients colonized with the respective strain (color in legend). C3 resistant bacteria were cultured from stool samples and analysed with MALDI-TOF MS.
For the gastrointestinal problems, the improvement in GSRS score during the trial was on average higher in the Probiotics and Saccharomyces group compared to placebo. This indicates less gastrointestinal discomfort when Saccharomyces or probiotics are added to an antibiotic treatment.
From the GSRS questionnaire, questions regarding constipation, diarrhea and loose stools the week before the visit were analyzed separately. These indicated a significant decline in constipation from visit 1 to visit 2 in the probiotics (62.5% to 25%) and Saccharomyces group (53.3% to 26.7%) (p=0.016 and p=0.043, respectively). Also 1 to 3 months after the antibiotics treatment, this decline was persistent in these groups with only 6.2% and 20% of patients suffering from constipation in the probiotics (p= 0.046) and Saccharomyces group (p=0.008) respectively, when following them from visit 1 to visit 3. A small difference between these two groups is the slightly higher (20% vs 6.2%) prevalence of constipation in the Saccharomyces group at the end of the trial (Figure 2).
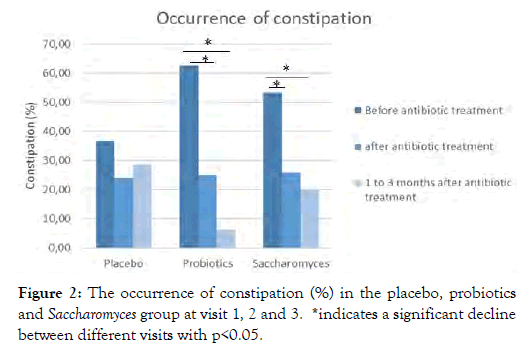
Figure 2: The occurrence of constipation (%) in the placebo, probiotics and Saccharomyces group at visit 1, 2 and 3. *indicates a significant decline between different visits with p<0.05.
Regarding Diarrhea, about 65.5%, 83.3% and 64.5% of patients reported no diarrhea after the antibiotics treatment in the placebo, probiotics and Saccharomyces group, respectively. These data indicate the lowest diarrhea severity in the probiotics group. At visit 3, no diarrhea was reported in the probiotics and Saccharomyces group while still 21.4% of patients taking placebo reported to have diarrhea the week before visit 3 (Figure 3).
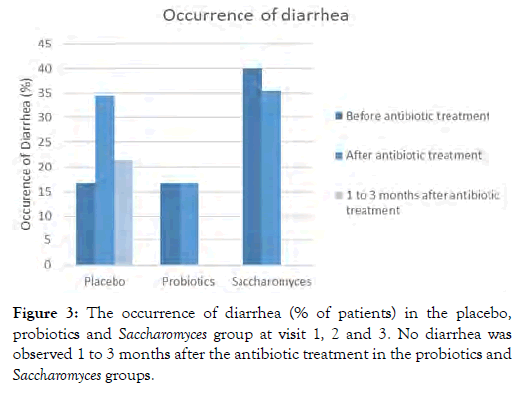
Figure 3: The occurrence of diarrhea (% of patients) in the placebo, probiotics and Saccharomyces group at visit 1, 2 and 3. No diarrhea was observed 1 to 3 months after the antibiotic treatment in the probiotics and Saccharomyces groups.
Additionally, when analyzing the question “did you suffer from loose stools last week?” from the daily bowel habit diary, a decline in patients suffering from loose stools when comparing visit 1 to visit 3 (31.2% to 6.2%, p=0.221) was seen in the probiotics group. The amount of patients suffering from loose stools was stable in the placebo and Saccharomyces group throughout the study (p=1.00) (Figure 4). When looking at individual scores, the prevention of loose stools in both the Saccharomyces and probiotics group during the antibiotics treatment was seen in 74.2-75% of patients.
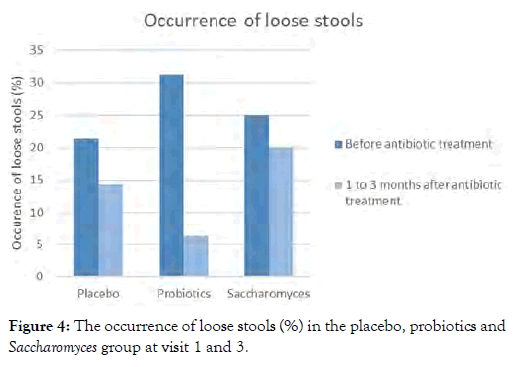
Figure 4: The occurrence of loose stools (%) in the placebo, probiotics and Saccharomyces group at visit 1 and 3.
A one-way ANOVA revealed no significant change in the number of defecations between the treatments (p=0.312). Patients in the placebo group had a significant increase in the number of defecations during the antibiotics treatment (2.07 ± 0.9) in comparison with 1 to 3 months after the antibiotics treatment (1.56 ± 0.54, p=0.018). The probiotics and Saccharomyces group showed a steady state in amount of defecations (p=0.694 and p=0.626, respectively). No difference was seen during and after the antibiotics therapy for difficulties to start the defecation in all groups. Regarding difficulties for ending the defecation, a higher occurrence is seen during the antibiotics treatment in the placebo group (p=0.286) while a rising trend is seen after the antibiotics treatment in the Saccharomyces group (p=0.325). In the probiotics group, a steady state was observed (p=0.787).
As shown by Ouwehand et al. the combination of the 4 probiotic strains lower the occurrence of antibiotic-associated diarrhea [9]. To the best of our knowledge, the additional effect of these strains in combination with Saccharomyces boulardii on the normalisation of the stool hasn’t been shown in any trial before.
The 4 specific probiotic strains in combination with Saccharomyces boulardii taken from the start of an antibiotic treatment until 2 weeks after cessation of the antibiotic treatment prevents from antibiotic-associated diarrhea and diminishes the colonision grade by opportunistic bacteria. Moreover the intake of the probiotics normalizes the stools during and after the antibiotic treatment, independent of the type of antibiotics used.
Citation: Vanheule G, Wieers G, Vynckier AK, Driessche MV (2020) Specific Probiotic Combination Exerts a Synergistic Effect against Antibiotic-Associated Diarrhea during and After an Antibiotic Therapy: A Randomized Double-Blind Placebo Controlled Trial. J Nutr Food Sci. 10:780. doi: 10.35248/2155-9600.20.10.780
Received: 17-Aug-2020 Accepted: 30-Aug-2020 Published: 07-Sep-2020 , DOI: 10.35248/2155-9600.20.10.1000780
Copyright: © 2020 Vanheule G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.