Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2014) Volume 4, Issue 5
Most of the residues after extracting juice from the citrus fruit are discarded, although they contain valuable substances such as flavonoids and essential oils. While most of the citrus flavonoids are present in the fruit peel, various valuable substances are also contained in the leaves. Moreover, the leaves and peels of citrus plants contain a large amount of moisture; hence, dewatering is essential for extracting valuable substances. Herein, we used liquefied dimethyl ether (DME) as the extractant for dewatering and extraction of valuable substances. With this process, >70% of the water from the wet samples was removed and valuable substances were isolated from the wet samples. The properties of the original and dewatered samples and the extract were evaluated using high-performance liquid chromatography. While the essential oils could be extracted, it was not possible to extract flavonoids by the liquefied DME method.
Keywords: Dimethyl ether; Citrus peel; Citrus leaves; Essential oils; Dewatering; Wet extraction
Nearly one million tons of citrus fruits are cultivated in Japan every year; these are used as the raw material for juice processing [1]. However, most of the pomace obtained after juice processing is disposed of, although it contains valuable substances such as flavonoids and essential oils [2]. Waste containing high moisture content is not only difficult to transport during disposal but also creates problems with regard to hygiene because it decomposes easily. Moreover, during the combustion process, the temperature of the combustion gases significantly drops inside the combustion furnace owing to the high moisture content of the diapers, and waste gases containing dioxins pollute the environment [3]. The disposal cost of the pomace is expensive because it contains a large amount of water [4]. Essential oils and citrus flavonoids are mainly contained in the pomace obtained from the peel and leaves of citrus [5]. These valuable substances are traded at a high price in the market. Essential oils are used in aromatherapy and high-quality perfumes. Citrus flavonoids are used as raw materials for health supplements; these flavonoids are also known to have hypolipidemic effects and apoptosis-inducing behavior in cancer cells [6]. Commercially, essential oils are obtained from the citrus peels by the cold press and steam distillation (SD) method [7,8]. However, the extraction yield by the cold press method is low, because large amounts of valuable oils remain in the residue [9]. On the other hand, pure essential oils can be obtained using the SD method. The distillation step is carried out over a temperature range of 130 to 150°C. The essential oils evaporate with the steam during the distillation process. However, the disadvantage of the SD method is that the quality of the obtained essential oils degrades as a result of the distillation heat [10]. Citrus flavonoids cannot be extracted using the SD method because it involves high boiling temperatures [11]. Additionally, a large amount of the residue with high water content is disposed of after the aforementioned processing methods. Therefore, the flavonoids are extracted using organic solvents such as menthol, dimethyl sulfoxide (DMSO), and hexane [12]. However, such extractions are toxic, expensive, and hazardous. Moreover, these methods require several preprocessing steps such as drying, grinding, and homogenizing the raw material [13]. Recently, alternative extraction methods are in demand in the food industry. Supercritical CO2 (SC-CO2) and liquefied dimethyl ether (DME) are receiving increased attention as the extraction solvent because of their desirable properties. SC-CO2 extraction has showed high selectivity and the possibility to fractionate the components based on temperature and pressure control [14]. However, it is necessary to use a special apparatus to withstand the high pressure of SC-CO2 [15]. DME has been developed as a synthetic fuel for use in both liquid and gaseous forms [16-18]. In China, DME is synthesized using small-scale coalfields of low commercial value and produced as a fuel at a cost equivalent to that of the imported liquefied petroleum gas. The standard boiling point of DME is -24.8°C and its saturated vapor pressure at 20°C is 0.51 MPa [19]. Because DME has weak hydrogen bonds, water dissolves into the liquefied DME to the extent of 7–8 wt% at room temperature. The quantity of gaseous DME dissolving in water is also low, and it can be easily separated from the water without distillation [20]. Furthermore, liquefied DME has low toxicity, and thus, could be investigated as a prospective solvent for food processing [21,22]. DME differs from typical ethers such as ethyl ether and does not form peroxides [23]. In addition, liquefied DME can extract not only water (dewatering) from brown coal [24], but also bio-crude (the organic components contained in the vegetal biomass) from biological materials such as microalgae [25]. The DME method exhibited robust dewatering ability and efficiency in removing the oils from the highmoisture orange peels [26]. Moreover, it can be operated at a relatively lower temperature and pressure, and easily separated from the extract at the ambient pressure [27]. In this work, water, essential oils, and flavonoids were extracted from the citrus pomace using liquefied DME. The peel and leaves of Citrus junos (Yuzu) and the peel of Citrus tangerine (Ponkan) were used as raw materials, and the dewatering rate and yield of essential oils and citrus flavonoids were investigated.
Outline of extraction method
Figure 1 shows a schematic of the laboratory-scale DME extraction apparatus. Two storage tanks, one containing liquefied DME (tank A, volume: 100 cm3; TVS-1-100, Taiatsu Techno Corp., Saitama, Japan) and an extraction column and the other holding the mixture of liquefied DME, water, and essential oils (tank B, HPG-96-3, Taiatsu Techno Corp., Saitama, Japan), were connected in series. The extraction column (diameter: 11.6 mm, length: 190 mm; HPG-10-5, Taiatsu Techno Corp., Saitama, Japan) was employed for the experiment. Additionally, bigger column (diameter: 35 mm, length: 190 mm; HPG- 96-3, Taiatsu Techno Corp., Saitama, Japan) was used as extractor because a large amount of essential oils were needed for the analysis. Citrus fruit peels comprise flavedo (exocarp) and albedo (endocarp). The essential oils are present in the oil glands of the flavedo. In these experiments, the peels of citrus fruits containing the albedo were used as the raw material. The essential oils in citrus peel were present in trace amounts (1–3%), the amount being smaller than the amount of water extracted from the citrus peel. The extraction of water and essential oils were performed three times, respectively. The test samples were loaded into the extraction column, and glass beads (diameter between 1.5 and 2.5 mm; BZ-2, ASONE Co., Inc., Osaka, Japan) were loaded at the top and bottom ends of the column [28]. In addition, for the removal of water, the loaded average amounts of Yuzu peel, Ponkan peel, and Yuzu leaves were 4.4 ± 0.1 g (82.7 wt%), 5.0 ± 0.1 g (72.4 wt%), and 2.6 ± 0.2 g (82.6 wt%), respectively. On the other hand, for the extraction of essential oils, the loaded average amounts of Yuzu and Ponkan peels were 20.1 ± 0.2 g (85.6 wt%) and 18.3 ± 0.3 g (72.4 wt%), respectively. Furthermore, the thickness of the Yuzu and Ponkan peels were approximately 5 mm and 2 mm, respectively. The values in the parentheses indicate the initial water contents, which were determined by drying at 107°C. The liquefied DME in tank A was controlled at 35 ± 2°C in a water bath, while the saturated vapor pressure of DME in tank A was 0.78 ± 0.03 MPa. The liquefied DME was supplied to the extraction column, and cooled down to room temperature using the long tube, which connected tank A and the extraction column. The temperature of the DME in the tube and extraction column was 15 ± 1°C, which was detected using an infrared thermometer. DME flowed owing to the pressure difference between tank A and the extraction column while the DME flow rate was maintained at 10 cm3 min–1 using a pressurereducing valve in the outlet of the extraction column. After the liquefied DME was passed through the extraction column at different time intervals, DME was evaporated by opening the pressure-reducing valve of tank B. The amount of the extracts remaining in tank B was equal to the total amount of essential oils and water extracted from the test samples. The extract was weighed after collection, and the yield of (1) essential oil and (2) water were determined using the equation shown below. In addition, the amount of citrus flavonoids in the water phase was analyzed using high-performance liquid chromatography (HPLC). The dry weights of the samples in the equation 1 were determined by drying at 107°C up to constant weight [26]. In other words, the dry weight was determined by subtracting both essential oil and water from the wet weight of the samples. Initial water amounts in the equation 2 were determined by difference between weight reduction by the drying and extracted essential oil amount.
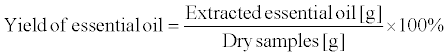 (1)
(1)
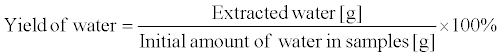 (2)
(2)
HPLC analysis
The amount of citrus flavonoids in the water phase was analyzed by high performance liquid chromatography (HPLC) using the external reference method. The standards, hesperidin (<91.3%) and naringin (<98.6%), were purchased from Kanto Chemical Co., Inc, Japan, while neohesperidin (<98.0%), nobiletin (<95.0%), tangeretin (<95.0%), HPLC-grade acetonitrile and acetic acid, used as the mobile phase, were purchased from Wako Pure Chemical Industries, Ltd., Japan. The flavonoids were identified by comparing their spectra and retention times with the standards. The content of each flavonoid was calculated from the integrated peak area of the sample and the corresponding standard. The flavonoid extracts were analyzed using an HPLC gradient system (LC-20AD, Shimadzu Corp., Kyoto, Japan) equipped with a diode array detector (SDP-M10A, Shimadzu Corp., Kyoto, Japan). The flavonoids were monitored at 285 nm. ODS column (Intertsil® ODS-3, GL Sciences Inc., Tokyo, Japan) was used for separation at 35°C. The mobile phase consisted of solvent A, 0.1% acetic acid in water, and solvent B, 0.1% acetic acid in acetonitrile (acetonitrile/water = 75/25, v/v). The flow rate was 1.0 mL/min. The gradient elution was carried out as follows: 0 min A–B (88:12); 5 min A–B (78:22); 15 min A–B (72:28); 22 min A–B (62:38); 32 min A–B (52:48); 37 min A–B (32:68); 42 min A–B (0:100); 45 min A–B (0:100); 50 min A–B (88:12); and 60 min, A–B (88:12) [29]. The yields of (3) citrus flavonoids were determined using the following equation.
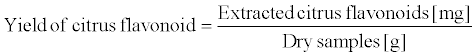 (3)
(3)
Steam distillation method
50 g of citrus peel was cut using scissors, and placed into a 1 L round bottom flask containing 500 mL of distilled water. Then, the flask was connected to a distilling receiver with Liebig condenser. Distillation was carried out for 4 h at atmospheric pressure. The essential oils evaporated with steam during the distillation process, and were separated from the condensates and collected in the distillate receiver. After reaching a certain volume, the water was refluxed in the distiller, and the distillation process continued [9].
Removal of water from citrus leaves and peel using liquefied DME
Figure 2 shows the amounts of water removed from the samples using liquefied DME. Here, owing to differing water contents in each sample, the DME consumption (abscissa) was expressed as the ratio between the consumption of DME and the total initial amount of water in the tested sample. The magnitudes of maximum dewatering in the Yuzu peel, Ponkan peel, and Yuzu leaves were as follows: 78.1 ± 1.0%, 76.2 ± 1.0%, and 83.9 ± 0.5%, respectively. As the Yuzu leaves were thin, the amounts of the extracted water were larger compared to those extracted from the other samples. Such large differences in the amounts of the extracted water among the tested samples were not confirmed. Therefore, it was possible to remove about 70–80% moisture from the raw material using liquefied DME. Moreover, the color of the extraction residue was relatively paler after DME extraction. Figure 3 shows the time-dependent change in the color of the extracted solution and that of the extraction residue compared to that of the Yuzu leaves. The color of the initial extract was dark green. The extract gradually changed into colorless by increasing the consumption of DME towards the end of the extraction process. On the other hand, the color of the residue turned to light green from dark green immediately after the extraction. Such change in the color intensity implied that the pigments of the sample were also extracted into the liquefied DME.
Comparison of yields of essential oils obtained by DME and SD methods
The extraction yields of essential oils from the samples by using the DME method were compared with those obtained by using the SD method, as shown in Figure 4. The extraction amounts of the essential oils were small. Figure 4 shows the final extraction amounts of the essential oils from the citrus peel. The black columns indicate the DME method extraction ratios based on the dry weight of the samples, while the white columns indicate the results by using the SD method. The numbers besides the superscripts ‘‘a’’ indicate the extraction yields obtained using DME relative to those achieved by using the SD method. In the case of extraction by using the SD method, the yields of essential oils from the Yuzu and Ponkan peels were 2.1% and 4.7%, respectively, which were typical for citrus peel [30,31]. Interestingly, in the case of extraction by using the DME method, the amounts of essential oils obtained were almost equal to those obtained by using the SD method. Furthermore, in the DME method, the extracts were not affected by thermal denaturation; hence, their compositions were close to that of the raw material. However, the Yuzu peel was thicker than the Ponkan peel; hence, the yield of the essential oils from the Yuzu peel was comparatively lower. In order to examine the effect of citrus peel thickness on the efficiency of the extraction of essential oils using the DME method, an additional experiment was carried out using Citrus grandis peel as the raw material. The reason for choosing Citrus grandis was its similarity to the Yuzu peel, making it possible to easily compare the extraction yield between the Citrus grandis and Yuzu peels. For the Citrus grandis peels, only the albedo parts were cut, leaving a 2-mm-thick peel. In another test experiment, we used a Citrus grandis peel containing the albedo parts, with a total thickness of 5 mm. For simplicity, the peel containing the thick albedo part is considered as untreated and the peel without the albedo parts is considered as pre-treated. The yield of the essential oils from the peel without treatment was 0.3 g/g-dry while the yield from the pre-treated sample was 3.4 g/g-dry. This result suggests that the albedo parts were preventing the extraction of the essential oils.
HPLC analysis of extracts obtained by DME method
Figure 5 shows the chromatograms of the citrus flavonoids extracted by using the liquefied DME method, which is typical for flavonoids [32]. Table 1 shows the quantitative results for these flavonoids. In the case of the Yuzu peel, naringin, hesperidin, and neohesperidin were extracted in 2.9 mg, 45.6 mg, and 1.4 mg, respectively. Hesperidin, nobiretin, and tangeretin were extracted from the Ponkan peel in 1.6 mg, 3.8 mg, and 1.2 mg, respectively. Hesperidin, neohesperidin, and phroletin were extracted from the Yuzu leaves in 0.3 mg, 17.9 mg, and 0.2 mg, respectively.
| Citrus flavonoid | Yield [mg/g-dry sample] | ||
|---|---|---|---|
| Yuzu peel | Ponkan peel | Yuzu leaves | |
| Naringin | 2.9 | - | - |
| Hesperidin | 45.6 | 1.6 | 0.3 |
| Neohesperidin | 1.4 | - | 17.9 |
| Phloretin | -a | - | 0.2 |
| Nobiretin | - | 3.8 | - |
| Tangeretin | - | 1.2 | - |
Table 1: Amount of citrus flavonoids extracted in water phase by DME extraction.
Citrus flavonoids are usually extracted from the citrus peel using 1:1 DMSO/methanol (v/v). The DMSO/methanol combination has high extraction ability, and is used for the quantitative analysis of flavonoids. While using DMSO/methanol as the extraction mixture, approximately 2% hesperidin per dry sample weight was extracted from the orange peel [13]. The extracted amounts of citrus flavonoids using liquefied DME were one-tenth of those obtained by using the DMSO/methanol method. These results suggested that the citrus flavonoids remained in the extracted residue. However, the use of DMSO for food processing is prohibited in Japan. In this study, it was possible to selectively extract essential oils from the citrus residues using liquefied DME. Therefore, there remain the citrus flavonoids in the extraction residue.
Water, essential oils, and citrus flavonoids were extracted from the citrus leaves and peels using liquefied DME. More than 70% water was removed from the test samples. The essential oils were also extracted in this process. In particular, almost the same amount of essential oils was extracted from the Ponkan peel. In addition, citrus flavonoids such as hesperidin, neohesperidin, nobiletin, tangeretin, and phloretin were extracted from all the samples. These flavonoids could not be extracted by the SD method. Therefore, DME extraction was highly effective in extracting water and essential oils from the citrus leaves and peels. These results suggested that the liquefied DME extraction method can be used to reduce the volume of citrus pomace, leading to a reduction in the disposal costs.