Journal of Food: Microbiology, Safety & Hygiene
Open Access
ISSN: 2476-2059
ISSN: 2476-2059
Research Article - (2023)Volume 8, Issue 7
Globally meat has always served the protein needs of varied populations. However, foodborne illnesses associated with the consumption of contaminated meat contribute significantly to reducing the efforts of health professionals and posing a great threat to health delivery systems. Antibiotic-resistant Salmonella enterica in meats is therefore of public health concern. This research was conducted to assess the prevalence and phenotypic antimicrobial resistance of Salmonella enterica isolated from raw meats, grilled Ready-To-Eat (RTE) meats, hands of meat sellers and their working tools in a one health context. The protocol in the United States Food and Drug Administration (USA- FDA) Bacteriological Analytical Manual was employed for the isolation of Salmonella enterica and the phenotypic antimicrobial resistance test was performed using the disk diffusion method. From a total of 200 meats and their related samples examined, 45 (23%) tested positive. It was observed that Salmonella enterica was highest in knives of fresh meat sellers (70%) whilst the tables of RTE meat sellers tested negative (0%). The results further indicated that isolates were highly resistant to teicoplanin (100%). Isolates exhibited a relatively high intermediate resistance to ciprofloxacin (78%) and ceftriaxone (33%). The isolates were however susceptible to chloramphenicol, trimethoprim, gentamicin, azithromycin, imipenem, amoxycillin and tetracycline, with susceptibility highest in chloramphenicol (90%). Also, 40% of the isolates exhibited multi-drug resistance, showing 22 different resistant patterns. The highest Multiple Antibiotic Resistance (MAR) index of the isolates recorded was 0.6 for isolates from RTE beef, chevon, chicken, guinea fowl and knife swab from RTE meat sellers. This study results indicate that Ready-To-Eat (RTE) meats, fresh meats and their related samples from Navrongo are a potential source of antibiotic-resistant Salmonella enterica with its likely transmission to humans.
RTE meat; Antibiotic resistance; Multiple Antibiotic Resistance (MAR) index; Humans
According to Ahmad, et al. global consumption of meat is high probably due to its good taste and high nutrient content [1]. Its high biological value also enables it to be easily used by the body of humans [2]. However, Ashwathi indicated that its consumption is associated with food borne illnesses, which significantly reduce the efforts of health personnel and threaten health delivery systems [3]. It is estimated that 600 million people fall ill for consuming contaminated food resulting in 420,000 deaths yearly [4]. Staphylococcus aureus, Escherichia coli, Salmonella enterica, Listeria monocytogenes, Campylobacter species and Clostridium species are among the predominant bacterial pathogens isolated from meats which are linked to several human illnesses and deaths annually [5,6].
European Food Safety Authority and European Centre for Disease Prevention and Control (EFSA and ECDC) has observed that from a total of 5,079 food/waterborne outbreaks reported, Salmonella species was the most common pathogen detected [7]. In addition, Salmonella species from meat, meat products, and eggs were the highest risk sources. Also, a review by Omer, et al. on bacterial foodborne outbreaks related to red meat and meat products between 1980 and 2015 showed that Salmonella species caused 21 outbreaks, mostly in Europe and the USA [8]. Salmonellae are responsible for millions of cases of enteric diseases, thousands of hospitalizations, and deaths worldwide annually [9]. In the USA, ninety-six (96) Salmonella outbreaks associated with beef were reported [10].
The increased emergence and wide spread of antibiotic-resistant foodborne bacteria is found to be associated with the intensive use of clinically important antimicrobials in human and veterinary medicine for therapeutics, prophylactics, and growth promotion [11]. Foods contaminated with antibiotic-resistant bacteria pose a major challenge to public health and have a negative impact on public health interventions [11]. Epidemiological data associated with the incidence of Salmonella and its antimicrobial resistance pattern is needed to develop an efficient mechanism toward its control at every level of the food processing and production chain to ensure food safety and public health [12].
Rane reported that street-vended foods are usually associated with foodborne diseases [13]. The meat vending (fresh and Ready-To- Eat (RTE)) trade is popular in Navrongo; making a significant contribution to the protein needs of the habitants. However, there is limited information on whether fresh and RTE meats produced in the municipality are contaminated by bacteria or contain resistant bacterial species. This scarcity of information has created a general perception that the meats might not be safe for consumption; causing significant economic losses for those who make a living from the trade. Therefore, to sustain the livelihoods of meat sellers, while ensuring health safety of consumers, this study investigated the phenotypic antimicrobial resistance of Salmonella enterica isolated from fresh and RTE meats, hands of meat sellers and their working tools in one health concept.
Study area
The study was conducted in Navrongo municipal, Ghana. The municipality is, adjacent to the border with Burkina Faso at Paga and covers a total land area of 865 square kilometres. The population of the municipality according to 2021 population and housing census stands at 99,895 with 48,658 males and 51,237 females [14]. Navrongo is an important market town, known for its cathedral and its grotto and located at 11°10’ and 10°3’ North and longitude 10°1’ West [15].
Study design and sample collection
Simple random sampling was employed to identify fresh and RTE meat sellers. A total of 200 fresh and RTE meat samples made up of beef (fresh=10; RTE=10), chevon (fresh=10; RTE=10), chicken (fresh=10; RTE=10), guinea fowl (fresh=10; RTE=10), mutton (fresh=10; RTE=10), and pork (fresh=10; RTE=10), sellers’ hands (Fresh=10; RTE=10), Sellers’ tables (Fresh=10; RTE=10), sellers’ knives (fresh=10; RTE=10) and sellers’ utensils (fresh=10; RTE=10) were randomly collected aseptically from the meat sellers in the municipality from February to September, 2022 and examined for the presence of Salmonella enterica.
Isolation of Salmonella enterica
Antibiotic resistance test
Standardized single disk method was used for the antibiotic-resistant test [17]. The Salmonella enterica isolates were examined against Amoxycillin 30 µg (A), Azithromycin 15 µg (Ath), Ceftriaxone 30 µg (Cro), Chloramphenicol 30 µg (C), Ciprofloxacin 5 µg (Cip), Gentamicin 10 µg (Gm), Trimethoprim 2.5 µg ('Tm), Tetracycline 30 µg (T), Teicoplanin (Tec) and Imipenem 10 µg (IMI) antibiotics.
Distribution of Salmonella enterica in the samples
The distribution of Salmonella enterica isolates in the various RTE meats, fresh meats and their related samples examined are shown in Table 1. Knife swab from fresh meat seller was the most contaminated (70%), followed by fresh beef (50%) and knife swab from RTE meat sellers (50%), whilst fresh chicken, fresh mutton, RTE pork, and hand swab from fresh meat sellers recorded the least contamination of 10% each. Fresh chevon, utensil swab from fresh meat sellers, and table swab from RTE meat sellers were not contaminated at all with Salmonella enterica.
| Sample source | Number of samples tested | Number of samples positive | Prevalence (%) |
|---|---|---|---|
| Fresh beef | 10 | 5 | 50 |
| Fresh chicken | 10 | 1 | 10 |
| Fresh guinea fowl | 10 | 2 | 20 |
| Fresh chevon | 10 | 0 | 0 |
| Fresh mutton | 10 | 1 | 10 |
| Fresh pork | 10 | 4 | 40 |
| RTE beef | 10 | 4 | 40 |
| RTE chicken | 10 | 2 | 20 |
| RTE guinea fowl | 10 | 2 | 20 |
| RTE chevon | 10 | 2 | 20 |
| RTE mutton | 10 | 3 | 30 |
| RTE pork | 10 | 1 | 10 |
| Knife swab from fresh meat seller | 10 | 7 | 70 |
| Table swab from fresh meat seller | 10 | 3 | 30 |
| Hand swab from Fresh meat seller | 10 | 1 | 10 |
| Utensil swab from fresh meat seller | 10 | 0 | 0 |
| Knife swab from RTE meat seller | 10 | 5 | 50 |
| Table swab from RTE meat seller | 10 | 0 | 0 |
| Hand swab from RTE meat seller | 10 | 1 | 10 |
| Utensil swab from RTE meat seller | 10 | 1 | 10 |
| Overall | 200 | 45 | 22.5 |
Table 1: The distribution of Salmonella enterica in the samples.
Antimicrobial resistance of the Salmonella enterica isolates
The antibiotic resistance of the isolates from fresh meats, RTE meats and their related samples is shown in the Figure 1. The results indicate that isolates were highly resistant to teicoplanin (100%). Isolates exhibited a relatively high intermediate resistance to ciprofloxacin (78%) and ceftriaxone (33%). The isolates were however susceptible to chloramphenicol, trimethoprim, gentamicin, azithromycin, imipenem, amoxycillin and tetracycline, with susceptibility highest in chloramphenicol (90%).
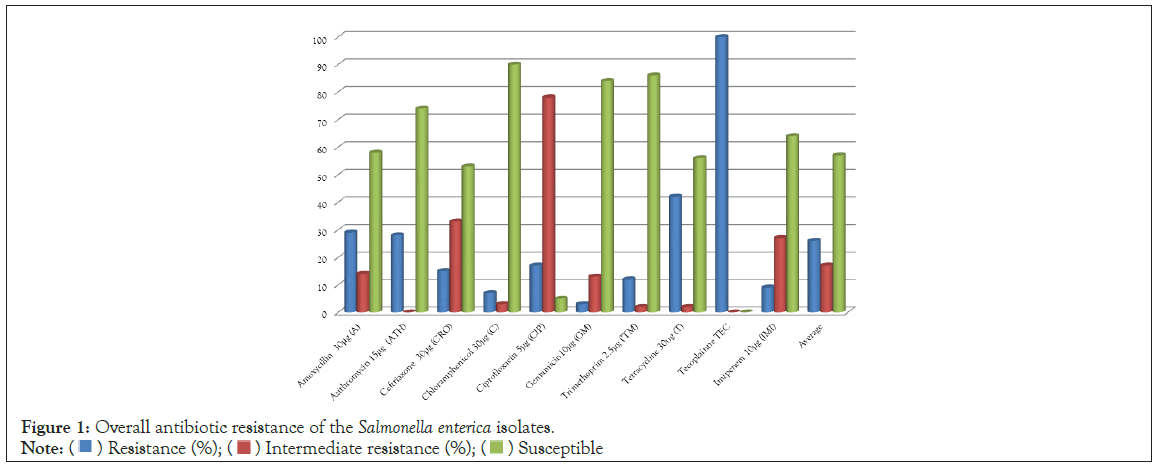
Figure 1: Overall antibiotic resistance of the Salmonella enterica isolates.
Note:  Resistance (%);
Resistance (%);  Intermediate resistance (%);
Intermediate resistance (%);  Susceptible
Susceptible
The phenotypic antimicrobial resistance of Salmonella enterica isolated from raw meats, RTE meats, hands of meat sellers and their working tools can be found in Table 2. It was observed that Salmonella enterica isolates of fresh meats, RTE meats, human hands and working tools’ origin were all highly resistant to teicoplanin (100%) with very high intermediate resistance to ciprofloxacin (≥ 62.5%). However, all the isolates were very susceptible to trimethoprim (≥ 81%) and gentamicin (≥ 64%).
| Antimicrobial | Samples from fresh meat (13 isolates) |
RTE Meat (14 isolates) |
Human Hands (2 isolates) |
Working Tools (16 isolates) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | R (%) | I (%) |
S (%) | |
| Amoxycillin 30 μg (A) | 39 | 0 | 62 | 50 | 7 | 43 | 0 | 50 | 50 | 25 | 0 | 75 |
| Azithromycin 15 µg (Ath) | 23 | 0 | 77 | 21 | 0 | 79 | 50 | 0 | 50 | 13 | 0 | 88 |
| Ceftriaxone 30 µg (Cro) | 7.7 | 31 | 62 | 21 | 43 | 36 | 0 | 50 | 50 | 31 | 6.3 | 63 |
| Chloramphenicol 30 µg (C) | 15 | 0 | 85 | 14 | 7.1 | 79 | 0 | 0 | 100 | 0 | 6.3 | 94 |
| Ciprofloxacin 5 µg (Cip) | 23 | 69 | 8 | 21 | 79 | 0 | 0 | 100 | 0 | 25 | 62.5 | 12.5 |
| Gentamicin 10 µg (Gm) | 0 | 15 | 85 | 7.1 | 29 | 64 | 0 | 0 | 100 | 6.3 | 6.3 | 88 |
| Trimethoprim 2.5 µg (Tm) | 0 | 7.7 | 92 | 29 | 0 | 71 | 0 | 0 | 100 | 19 | 0 | 81 |
| Tetracycline 30ug (T) | 31 | 0 | 69 | 57 | 7.1 | 36 | 50 | 0 | 50 | 31 | 0 | 69 |
| Teicoplanin (Tec) | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 | 100 | 0 | 0 |
| Imipenem 10µg (Imi) | 7.7 | 23 | 69 | 29 | 14 | 57 | 0 | 50 | 50 | 0 | 19 | 81 |
| Average | 25 | 15 | 61 | 35 | 19 | 46 | 20 | 25 | 55 | 25 | 10 | 65 |
Note: Fresh meats: Fresh beef, chevon, chicken, guinea fowl, pork, and mutton; RTE, Ready-to-eat meats: RTE beef, chevon, chicken, guinea fowl, pork, and mutton; R, Resistant; I, Intermediate resistance; S, Susceptible; Human hand samples: Hand swab from fresh and RTE meat sellers; Working tools: Swab samples from knives, tables and utensils of fresh and RTE meat sellers.
Table 2: Phenotypic antibiotic resistance of Salmonella enterica isolates from fresh meats, RTE meats, hands and working tools of meat sellers.
Antibiotic resistance profile and MAR index of individual Salmonella enterica isolates
Table 3 shows the Multiple Antibiotic Resistance (MAR) index and antimicrobial resistance pattern of individual Salmonella enterica isolates from fresh meats, RTE meats and their related samples. The MAR index of the Salmonella enterica isolates ranged between 0.1 and 0.6 showing 22 different resistant patterns. The resistance to Tec (Teicoplanin) and Tec-T (Teicoplanin-Tetracycline) were found in thirteen and seven different Salmonella enterica isolates, respectively whereas the resistance patterns A-Tec (Amoxycillin-Teicoplanin) and Ath-Tec (Azithromycin-Teicoplanin) were observed for three isolates each. Isolates from RTE beef (RB10), RTE chevon (Rch3), RTE chicken (RC6), RTE guinea fowl (RG2) and knife swab from RTE meat seller (RK4) recorded the highest number of resistance to six antibiotics each, thus AAthTecTCTm, ATecTCCroImi, CipAAthTecCroTmImi, CipATecTTmImi, and AAthTecTCroTm, respectively. Isolates from knife swab from fresh meat seller’s origin (FK8) showed resistance to five antimicrobials (ATecTCroTm). In addition, 18 isolates exhibited multidrug resistance (resistance to three or more different classes of antimicrobials) representing 40%. Figure 2 shows inhibition zones of some antibiotics measured.
| Sample code | Sample source | No. of Antibiotics | Antibiotics resistant profile | MAR Index |
|---|---|---|---|---|
| RB10 | RTE beef | 6 | AAthTecTCTm | 0.6 |
| Rch3 | RTE chevon | 6 | ATecTCCroImi | 0.6 |
| RC6 | RTE chicken | 6 | CipAAthTecCroTmImi | 0.6 |
| RG2 | RTE guinea fowl | 6 | CipATecTTmImi | 0.6 |
| RK4 | Knife swab from RTE meat seller | 6 | AAthTecTCroTm | 0.6 |
| FK8 | Knife swab from fresh meat seller | 5 | ATecTCroTm | 0.5 |
| FB9 | Fresh beef | 4 | CipATecT | 0.4 |
| FM5 | Fresh mutton | 4 | ATecCCro | 0.4 |
| FP10 | Fresh pork | 4 | CipAAthTec | 0.4 |
| FP6 | Fresh pork | 4 | ATecCImi | 0.4 |
| RB8 | RTE beef | 4 | CipAthTecCro | 0.4 |
| Rch2 | RTE chevon | 4 | ATecTmImi | 0.4 |
| FK4 | Knife swab from fresh meat seller | 4 | CipTecTCro | 0.4 |
| FT6 | Table swab from fresh meat seller | 4 | CipAthTecCro | 0.4 |
| FT9 | Table swab from fresh meat seller | 4 | ATecTTm | 0.4 |
| RM4 | RTE mutton | 3 | ATecGm | 0.3 |
| FK10 | Knife swab from fresh meat seller | 3 | CipTecGm | 0.3 |
| FT3 | Table swab from fresh meat seller | 3 | CipTecCro | 0.3 |
| FB1 | Fresh beef | 2 | CipTec | 0.2 |
| FB10 | Fresh beef | 2 | AthTec | 0.2 |
| FCH3 | Fresh Chevon | 2 | AthTec | 0.2 |
| FG2 | Fresh guinea fowl | 2 | TecT | 0.2 |
| FG3 | Fresh guinea fowl | 2 | TecT | 0.2 |
| FP7 | Fresh pork | 2 | ATec | 0.2 |
| RB2 | RTE beef | 2 | TecT | 0.2 |
| RM2 | RTE mutton | 2 | TecT | 0.2 |
| RM5 | RTE mutton | 2 | ATec | 0.2 |
| RP5 | RTE pork | 2 | TecT | 0.2 |
| FH4 | Hand swab of fresh meat seller | 2 | TecT | 0.2 |
| RH4 | Hand swab of RTE meat seller | 2 | AthTec | 0.2 |
| RK3 | Knife swab from RTE meat seller | 2 | TecT | 0.2 |
| RK5 | Knife swab from RTE meat seller | 2 | ATec | 0.2 |
| FB1 | Fresh beef | 1 | Tec | 0.1 |
| FB3 | Fresh beef | 1 | Tec | 0.1 |
| FP5 | Fresh pork | 1 | Tec | 0.1 |
| RB6 | RTE beef | 1 | Tec | 0.1 |
| RC2 | RTE chicken | 1 | Tec | 0.1 |
| RG1 | RTE guinea fowl | 1 | Tec | 0.1 |
| FK1 | Knife swab from fresh meat seller | 1 | Tec | 0.1 |
| FK5 | Knife swab from fresh meat seller | 1 | Tec | 0.1 |
| FK1 | Knife swab from fresh meat seller | 1 | Tec | 0.1 |
| FK9 | Knife swab from fresh meat seller | 1 | Tec | 0.1 |
| RK10 | Knife swab from RTE meat seller | 1 | Tec | 0.1 |
| RK6 | Knife swab from RTE meat seller | 1 | Tec | 0.1 |
| RU1 | Utensil swab from RTE meat seller | 1 | Tec | 0.1 |
Table 3: Antibiotic resistance profile and MAR index of individual isolates.
Figure 2: Plates showing zones of inhibition (clear zones around each antibiotic disc).
Antibiotic resistance profile and MAR index of individual Salmonella enterica isolates
Observing meat safety practices is the key to reducing the incidence of foodborne diseases associated with contaminated meats. Salmonella enterica was detected in 22.5% of the meat and its related samples. According to Centre for Food Safety, in 25 g of RTE meat sample, Salmonella should not be detected at all [18]. Therefore, the fourteen (14) RTE meat samples in the current work can be described as unsatisfactory. This contamination may have occurred due to cross-contamination as a result of improper handling after grilling. However, 77% of the RTE meats in this study were satisfactory and safe to eat regarding Salmonella enterica contamination and subsequent infection. The relatively low rate of Salmonella enterica contamination of RTE meats in this study could be attributed to the meat safety knowledge of RTE meat sellers as reported by Aduah, et al. [19]. The 22% occurrence in fresh and 23% in RTE samples in the current study is lower than a prevalence of 64.9% in chicken by Mokgophi, et al. and 57.01% observed in beef and its related samples by Adzitey, et al. [20,21] in Techiman, Ghana, but higher than findings of Aduah, et al. [19] who reported 6.0% occurrence of Salmonella enterica in different types of RTE meats in Bolgatanga and Cabedo et al. who reported Salmonella enterica prevalence of 1.5% in frozen chicken croquettes from Spain [22].
Antimicrobial resistance of the Salmonella enterica isolates
The results showed that all the isolates were highly resistant to teicoplanin but susceptible to chloramphenicol, trimethoprim, gentamicin, azithromycin, imipenem, amoxycillin, tetracycline and ceftriaxone. This study results concord with findings of Terentjeva, et al. who reported that Salmonella isolates from meat and meat products were highly susceptible to azithromycin (100%) and Harakeh, et al. who found 100% resistance to teicoplanin by Salmonella species isolated from meat-based fast foods but contrary to Aduah, et al. [19] who found a higher resistance to azithromycin (83.33%), amoxycillin (66.67%) and tetracycline (50.00%) from RTE meats [23, 24]. The results also differ from findings of Khaitsa, et al. who indicated that 86.0% of Salmonella isolates from RTE meats exhibited multidrug resistance as against 40.0% in this study [25].
The study showed sporadic Salmonella enterica contamination in the meats and related samples. This demonstrates that they could serve as a reservoir of resistant and pathogenic Salmonella enterica, which harbourvirulencefactors and exhibit resistance tovarious antibiotics. The presence of many MAR Salmonella enterica isolates among fresh, RTE meats and their related samples in this study indicates that the imprudent use of antibiotics on farms could contribute to the increasing development and spread of antibiotic resistance in food animals and animal food products. Therefore, the initial steps to reduce and control Salmonella enterica contamination of meat should be taken at the farm level by developing and implementing an Antimicrobial Resistance National Strategy Framework with the strategic objective of promoting the appropriate use of antibiotics in humans and animals using regulations. There is also the need for regular surveillance of Salmonella enterica incidence in meats and related sources, especially in grilled RTE meats to ensure a continuous supply of safety foods.
Citation: Aduah M, Adzitey F, Ekli R, Teye AG, Huda N (2023) Phenotypic Antimicrobial Resistance of Salmonella enterica Isolated from Meats, Humans and Working Tools. J Food Microbial Saf Hyg. 8:248.
Received: 01-Sep-2023, Manuscript No. JFMSH-23-25389; Editor assigned: 04-Sep-2023, Pre QC No. JFMSH-23-25389 (PQ); Reviewed: 18-Sep-2023, QC No. JFMSH-23-25389; Revised: 25-Sep-2023, Manuscript No. JFMSH-23-25389 (R); Published: 02-Oct-2023 , DOI: 10.35248/2476-2059.23.8.248
Copyright: © 2023 Aduah M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.