Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2012) Volume 0, Issue 0
The present day approach is value addition of convenience foods by incorporating strengthful ingredients, which offer either therapeutic or preventive medicinal health benefits, in addition to provision of their basic nutrition. Flaxseed is one of the nutritional crops as it is rich in omega-3 fatty acids, proteins, dietary fiber and phytochemicals. Central Composite Rotatable Design (CCRD) was used to optimize process parameters for development of flaxseed and defatted flaxseed based pasta products, by response surface methodology with three variables at five levels. The low and high levels of the variables were 70 to 90g for semolina; 10 and 30g for flaxseed or defatted flaxseed; 15 and 20% water (% of amount of semolina and flaxseed) for flaxseed based pasta, and 20 and 30% water (% of amount of semolina and flaxseed) for defatted flaxseed based pasta. Pasta was made by extrusion using a spiral die in pilot plant pasta making machine (D-35, La Parmigiana), followed by convective dehydration to a final moisture content of 7-8% (w.b) to make it shelf stable product. The optimum values for process variable for maximum possible gelatinization, color parameter (L*), overall acceptability, minimum free fatty acid, and color parameter (a*) are semolina 90g, flaxseed 15g and water* 20 for flaxseed based pasta, and semolina 87.79g, flaxseed 15g and water* 30 for defatted flaxseed based pasta.
Keywords: Flaxseed; Defatted flaxseed; Pasta; Gelatinization; Optimization; ALA Content (alpha-linolenic acid)
Nowadays, interest has led to produce a wide variety of foods referred to as functional foods and nutraceuticals because these type of foods offer either therapeutic or preventive medicinal health benefits, in addition to provision of their basic nutrition. Flaxseed is the small flat oval seed from flax (Limum usitatissimum), which has been gaining popularity as a functional food because of its high content of biologically active compounds, their health benefits, and disease preventive properties [1]. The Latin name of flaxseed is Linum usitatissimum, which means “very useful”. Flax is a nutrition crop because of the high content of alpha-linolenic acid in the flaxseed oil, its dietary fiber, high quality proteins and lignans as secondary metabolite. Traditionally, industrial applications of flaxseed include as linen textile or drying oil in paints, varnishes, and linoleum [2]. Medicinal applications of flaxseed as antitumoral and anti-inflammatory remedy were also known since antiquity [3,4]. The use of flaxseed in food can increase the content of a-linolenic acid and long chain ω-3 fatty acids in both blood plasma and erythroplastid fat, and reduce the content of cholesterol [5]. Omega-3 fatty acids also have anti-inflammatory, anti-thrombotic, and anti-arrhythmic properties [6]. Additionally, insoluble fibers present in flaxseed affects plasma lipids and lignans such as Secoisolariciresinol Diglucoside (SDG), which exhibit protective effects against hormonerelated types of cancer like breast cancer, and against non-hormone related colon cancer [7-11]. Functional properties of flaxseed proteins, including hypotriglyceridemic and hypocholesterolemic effects are reported to be superior to those of commonly known soy proteins [12].
Recent food applications of flaxseed oil include their use in salad dressings, as a partial substitute for milk fat in ice cream desserts [13,14]. The flaxseed fibre has a wide range of applications in various areas such as textile, paper, and biocomposite manufacturing [15]. Flax gums are used as a food ingredient to improve texture, to prevent syneresis in dairy products, and can play the role of the stabiliser in vegetable and fruit juices [16]. Flaxseed proteins have been assessed as technofunctional ingredients in many food formulations, such as bakery products and pastries, meat emulsions, sauces and ice creams [17-19]. Recently, efforts have been made for utilizing flaxseed or understanding its functional properties, but little work had been referred with reference to systematic development of flaxseed based products.
In past decade, pasta products have given a new dimension in the convenience foods. Though traditionally, wheat based noodles were in practice, in recent years with the technological development, many products of commerce have taken its place. The value addition by incorporation of strengthful ingredients or fortification with nutrients is the present day approach. The present study was conducted for development and optimization of value added flaxseed and defatted flaxseed based pasta products, using response surface methodology.
Processing of flaxseed
Flaxseed was purchased from Grain Market, Sector- 26, Chandigarh. Flaxseeds were cleaned, milled in a flour mill, and sieved. Defatted flaxseed was obtained by extracting milled flaxseed with chloroform: methanol (2:1) solvent mixture. Sample and solvent is mixed in 2:1 and stirred for 1h in a shaker. The micelle was removed by vacuum filtration and extraction was repeated twice. After complete extraction, the resulting meal was dried in a tray drier at 50°C, till the material is free of solvent.
Chemical analysis
The flaxseed and defatted flaxseed flour was analyzed for moisture, crude protein (N×6.25), fat, ash and crude fiber contents, according to standard method (AOAC) [20]. The carbohydrate content was calculated by difference method. Insoluble and soluble dietary fiber was estimated by enzymatic method of Asp et al. [21]. All proximate analyses were carried out in triplicate and reported in percentages. Color values (L*, a*, b*) of pasta was measured by Hunter Color Lab difference meter (L* means lightness, with 100 for white, 0 for black; a* indicates redness when positive, and greenness when negative, b* indicates yellowness when positive, and blueness when negative), and free fatty acid (FFA, %) was determined, according to the method reported by Hautfenne [22] for fats and oils.
Gelatinization (%)
The gelatinization (%) was measured according to the method reported by Birch and Priestley [23]. The cooked pasta was dried in oven at 58°C, and ground through the sieve (80 mesh). The sample (0.2 g) was prepared in 125 ml Erlenmeyer flasks; 98ml of distilled water and KOH (10 M) 2.0 ml was added and then mixed for 5 minutes, prior to centrifugation at 3000 rpm for 15 min. The supernatant (1.0 ml) was pipetted and added with hydrochloric acid (0.5M) 0.4 ml, followed by 10 ml of distilled water and 0.1 ml of iodine solution. The mixture was homogenized, then measuring the absorbance at 600 nm.
The gelatinization (%) of standard starch was prepared as in the same manner of sample to obtain the standard curve of rice and applied to calculate degree of gelatinization of sample.
Experimental design
Experiments were conducted according to Central Composite Rotatable Design (CCRD) [24], with three variables at five levels each, to optimize the process variables for development of flaxseed and defatted flaxseed based pasta products. The CCRD design predicts uniformly at all constant distances from their center points. The independent variables for development of flaxseed pasta were amount of semolina (g), flaxseed floor (g) and water (ml) (% of semolina (g) and flaxseed flour (g)) (Table 1), and for defatted flaxseed pasta were amount of semolina (g), defatted flaxseed floor (g) and water (ml) (% of semolina (g) and defatted flaxseed flour (g)) (Table 2). The water absorption power of flour mixture varied according to amount of flaxseed and semolina, so amount of water was calculated as percentage of amount of semolina and flaxseed. The dependent variables chosen were %free fatty Acid, %gelatinization, color parameter (L*, a*), and overall acceptability (OAA). Experimental design in uncoded form of process variables, along with results for optimization of process variables for flaxseed and defatted flaxseed pasta are given in table 3 and 4, respectively. The experiments were conducted randomly to minimize the effects of unexplained variability in the observed responses, because of external factors.
| Variable Levels | ||||||
|---|---|---|---|---|---|---|
| Process variable | Code | Coded Levels | ||||
| -1.68 | -1 | 0 | +1 | +1.68 | ||
| Semolina (g) | X1 | 63.18 | 70 | 80 | 90 | 96.82 |
| Flaxseed (g) | X2 | 3.18 | 10 | 20 | 30 | 36.82 |
| Water (% of X1+X2) | X3 | 13.3 | 15 | 17.5 | 20 | 21.71 |
Table 1: Experimental design in coded and uncoded form of process variables for development of flaxseed based pasta.
| Variable Levels | ||||||
|---|---|---|---|---|---|---|
| Process variable | Code | Coded Levels | ||||
| -1.68 | -1 | 0 | +1 | +1.68 | ||
| Semolina (g) | X1 | 63.18 | 70 | 80 | 90 | 96.82 |
| Defatted flaxseed (g) | X2 | 3.18 | 10 | 20 | 30 | 36.82 |
| Water (% of X1+X2) | X3 | 16.6 | 20 | 25 | 30 | 33.41 |
Table 2: Experimental design in coded and uncoded form of process variables for development of defatted flaxseed based pasta.
| Semolina (g) | Flaxseed (g) | Water* | % FFA | % Gelatinization | OAA | Colour (L*) | Colour (a*) |
|---|---|---|---|---|---|---|---|
| 70 | 10 | 15 | 1.385 | 48.628 | 7.265 | 62.99 | 3.002 |
| 90 | 10 | 15 | 1.316 | 58.886 | 8.165 | 61.778 | 2.739 |
| 70 | 30 | 15 | 3.532 | 55.732 | 6.815 | 73.111 | 3.666 |
| 90 | 30 | 15 | 3.43 | 69.26 | 6.995 | 71.558 | 3.481 |
| 70 | 10 | 20 | 1.392 | 52.856 | 7.335 | 62.05 | 3.102 |
| 90 | 10 | 20 | 1.325 | 62.17 | 8.215 | 62.728 | 2.813 |
| 70 | 30 | 20 | 3.651 | 63.714 | 7.025 | 70.045 | 3.687 |
| 90 | 30 | 20 | 3.577 | 73.114 | 7.145 | 71.29 | 3.348 |
| 63.18 | 20 | 17.5 | 3.127 | 48.172 | 6.715 | 61.439 | 3.464 |
| 96.82 | 20 | 17.5 | 2.86 | 70.342 | 7.215 | 63.897 | 3.044 |
| 80 | 3.18 | 17.5 | 0.83 | 61.886 | 8.315 | 61.365 | 2.3 |
| 80 | 36.82 | 17.5 | 4.49 | 73.714 | 7.465 | 77.389 | 3.75 |
| 80 | 20 | 13.29 | 2.58 | 49.2 | 7.215 | 67.772 | 3.353 |
| 80 | 20 | 21.7 | 2.61 | 62.056 | 7.315 | 66.907 | 3.367 |
| 80 | 20 | 17.5 | 2.87 | 63.376 | 7.565 | 67.47 | 3.581 |
| 80 | 20 | 17.5 | 2.42 | 59.142 | 7.415 | 67.195 | 3.38 |
| 80 | 20 | 17.5 | 2.91 | 60.064 | 7.265 | 67.03 | 3.3 |
| 80 | 20 | 17.5 | 2.48 | 63.738 | 7.735 | 65.313 | 3.569 |
| 80 | 20 | 17.5 | 2.97 | 59.668 | 7.135 | 65.242 | 3.447 |
| 80 | 20 | 17.5 | 2.38 | 63.94 | 7.615 | 67.24 | 3.566 |
* Water (ml)=% of Semolina (g)+Flaxseed (g)
Table 3: Central Composite Rotatable Design with experimental values of response variables for development of flaxseed based pasta.
| Semolina (g) | Defatted Flaxseed (g) | Wate* | % FFA | % Gelatinization | OAA | Colour (L*) | Colour (a*) |
|---|---|---|---|---|---|---|---|
| 70 | 10 | 20 | 0.306 | 57.4 | 7.35 | 71.88 | 2.712 |
| 90 | 10 | 20 | 0.266 | 73.72 | 8.25 | 70.668 | 2.449 |
| 70 | 30 | 20 | 0.866 | 63.1 | 6.9 | 82.001 | 3.376 |
| 90 | 30 | 20 | 0.84 | 80.5 | 7.08 | 80.448 | 3.191 |
| 70 | 10 | 30 | 0.347 | 71.96 | 7.42 | 70.94 | 2.812 |
| 90 | 10 | 30 | 0.299 | 84.38 | 8.3 | 71.618 | 2.623 |
| 70 | 30 | 30 | 0.89 | 75.42 | 7.11 | 78.935 | 3.397 |
| 90 | 30 | 30 | 0.82 | 87.86 | 7.23 | 80.18 | 3.058 |
| 63.18 | 20 | 25 | 0.778 | 58.46 | 6.8 | 71.329 | 3.174 |
| 96.82 | 20 | 25 | 0.767 | 84.12 | 7.3 | 73.787 | 2.95 |
| 80 | 3.18 | 25 | 0.173 | 69.14 | 8.3 | 69.55 | 2.21 |
| 80 | 36.82 | 25 | 0.995 | 80.24 | 7.65 | 86.279 | 3.51 |
| 80 | 20 | 16.59 | 0.286 | 69.84 | 7.3 | 75.662 | 3.063 |
| 80 | 20 | 33.41 | 0.318 | 89.66 | 7.4 | 74.797 | 3.077 |
| 80 | 20 | 25 | 0.546 | 83.01 | 7.65 | 75.36 | 3.291 |
| 80 | 20 | 25 | 0.521 | 85.5 | 7.5 | 75.085 | 3.09 |
| 80 | 20 | 25 | 0.467 | 82.66 | 7.35 | 74.98 | 3.01 |
| 80 | 20 | 25 | 0.493 | 82.56 | 7.82 | 73.013 | 3.279 |
| 80 | 20 | 25 | 0.461 | 85.62 | 7.22 | 73.132 | 3.157 |
| 80 | 20 | 25 | 0.421 | 83.46 | 7.7 | 75.23 | 3.276 |
* Water (ml)=% of Semolina (g)+Defatted Flaxseed (g)
Table 4: Central Composite Rotatable Design with experimental values of response variables for development of defatted flaxseed based pasta.
Preparation of pasta
Flaxseed flour, semolina and water were weighed according to the experimental design (Table 3). Salt (3%) and lemon juice (8%) was mixed with pre-weighed amount of water to enhance the flavour of pasta. Dough was prepared by mixing all the ingredients, and rested for half an hour. Then, it was extruded using a spiral die in pilot plant pasta making machine (D-35, La Parmigiana). Extruded samples were then dried in a tray drier at 60°C to a final moisture content of 7-8 % (w.b). Dried samples were packed in paper-foil-polythene laminate pouch and stored for analysis at room temperature. Similar procedure was repeated using defatted flaxseed floor for preparation of defatted flaxseed pasta (Table 4).
Sensory evaluation
A known amount of extruded samples (25 g) was taken and added to boiling salted water pan, while continuing the heating for 10-12 min. After every minute, a piece of pasta was held between two glass plates and was compressed. Optimum cooking of pasta was established, when no white core was observed after compressing. Organoleptic quality of cooked flaxseed and defatted flaxseed pasta was determined with the help of a 10-member consumer panel, using a 9-point Hedonic scale. The organoleptic attributes included appearance, flavour, odor, taste and overall acceptability. The average scores of all 10 panelists were computed for different characteristics.
Statistical analysis and optimization
The second-order polynomial equation was fitted to the experimental data of each dependent variable
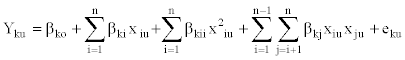 (1)
(1)
Where Yku=response variable (Y1u=% free fatty acid, Y2u=% gelatinization, Y3u=overall acceptability of cooked pasta, Y4u=color parameter (L*), Y5u=color parameter (a*)), xiu represents the coded independent variables (x1u=semolina (g), x2u=flaxseed (g) or defatted flaxseed (g), x3u=water (ml) (% of x1u and x2u). Coefficient ßko is the value of fitted response at the center point design, i.e. point (0,0,0), and ßki, ßkii and ßkj are the linear, quadratic and interaction regression coefficients, respectively.
Optimization of the process variable for development of flaxseed and defatted flaxseed based pasta was aimed at finding the levels of independent variables, viz. semolina, flaxseed or defatted flaxseed and water which would result in minimum free fatty acid and color parameter (a*) and maximum degree of gelatinization, overall acceptability and color parameter (L*) of pasta. Response Surface Methodology (RSM) was applied to the experimental data using Design Expert statistical software (version 6.0.1, Stat-Ease, Inc., Minneapolis, MN), to evaluate the effect of various process parameters on measured responses at 5% level of significance. Analysis of variance (ANOVA) was conducted to fit the model represented by Eq. (1) to examine the statistical significance of the model terms. The adequacy of the models was determined using model analysis, lack-of-fit tests, and R2 (coefficient of determination). The value of β coefficient was used to compare the relative contribution of each independent variable in the prediction of the dependent variable. Higher the positive value of β of a parameter, higher would be the effect of that parameter, and viceversa. The response surface plots were generated for interaction of any two independent variables, while holding the value of third-and fourthvariable as constant (at the central value). The same software (version 6.0.1, Stat-Ease, Inc., Minneapolis, MN) was used for the generation of response surface plots, superimposition of contour plots, and optimization of process variables.
Proximate composition
The fat content of flaxseed flour is found to be above 40%, and after solvent extraction, the fat content reduced to less than 1% (Table 5). The protein, crude fiber and ash content of defatted flaxseed are higher than that of flaxseed flour, which is due to removal of fat. The results of chemical components in flaxseed flour are within the amounts reported by Mueller, Mazza, Bozan and Temelli et al. [4,25,26]. The moisture content of flaxseed flour is found to higher than reported by Bozan and Temelli [26], which may be due to climatic changes.
| Constituents | Flaxseed Flour | Defatted Flaxseed Flour |
|---|---|---|
| Moisture (w.b) | 8.78±0.02 | 7.83±0.1 |
| Fat | 41.78±0.1 | 0.94±0.06 |
| Protein | 22.59±0.12 | 38.16±0.15 |
| Crude fiber | 9.687±0.2 | 15.14±0.21 |
| Ash | 3.48±0.2 | 5.86±0.2 |
| Carbohydrates | 13.683±0.4 | 31.97±0.3 |
| Soluble dietary Fiber | 14.43±0.2 | 21.34±0.2 |
| Insoluble dietary fiber | 30.62±0.2 | 41.67±0.2 |
Table 5: Proximate composition of flaxseed flour and defatted flaxseed flour.
Diagnostic checking of fitted models and response surfaces
The results of second-order response surface model (eq. 1) in the form of ANOVA are given in table 5-9 for development of flaxseed and defatted flaxseed based pasta. The results indicated that the fitted quadratic models accounted for more than 95% of the variation in the experimental data (R2>0.90), which were significant at 5% level of significance (p<0.05).
| Source | df | Flaxseed flour based pasta | Defatted flaxseed based pasta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SS | F Value | p-value | β | SS | F Value | p-value | ||
| Model | 9 | 16.605 | 27.459 | <0.0001 | 1.198 | 82.311 | <0.0001 | ||
| Constant | 1 | 2.681 | - | - | - | 0.484 | |||
| Semolina | 1 | -0.056 | 0.042 | 0.631 | 0.4454 | -0.015 | 0.003 | 1.857 | 0.2029 |
| Flaxseed | 1 | 1.093 | 16.316 | 242.819 | <0.0001 | 0.262 | 0.939 | 580.443 | <0.0001 |
| Water | 1 | 0.024 | 0.008 | 0.120 | 0.7357 | 0.010 | 0.001 | 0.787 | 0.3959 |
| Semolina2 | 1 | 0.050 | 0.036 | 0.532 | 0.4826 | 0.106 | 0.163 | 100.903 | <0.0001 |
| Flaxseed2 | 1 | -0.068 | 0.067 | 0.995 | 0.3421 | 0.040 | 0.023 | 14.091 | 0.0038 |
| Water2 | 1 | -0.091 | 0.120 | 1.780 | 0.2118 | -0.060 | 0.052 | 32.014 | 0.0002 |
| Semolina x Flaxseed | 1 | -0.005 | 0.000 | 0.003 | 0.9576 | -0.001 | 0.000 | 0.005 | 0.9453 |
| Semolina x Water | 1 | 0.004 | 0.000 | 0.002 | 0.9682 | -0.006 | 0.000 | 0.209 | 0.6573 |
| Flaxseed x Water | 1 | 0.031 | 0.008 | 0.116 | 0.7402 | -0.009 | 0.001 | 0.379 | 0.552 |
| Residual | 10 | 0.672 | 0.016 | ||||||
| Lack of Fit | 5 | 0.302 | 0.715 | 0.6862 | 0.006 | 0.605 | 0.7027 | ||
| Pure Error | 5 | 0.370 | 0.010 | ||||||
| R2 | 0.96111 | 0.98668 | |||||||
| Adjusted R2 | 0.92611 | 0.97469 | |||||||
Table 6: ANOVA showing the variables as a linear, quadratic, and interaction terms on % free fatty acid and coefficients for the prediction models.
| Source | df | Flaxseed flour based pasta | Defatted flaxseed based pasta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SS | F Value | p-value | β | SS | F Value | p-value | ||
| Model | 9 | 257.152 | 25.907 | <0.0001 | 437.209 | 142.621 | <0.0001 | ||
| Constant | 1 | 30.831 | 41.907 | ||||||
| Semolina | 1 | 2.943 | 118.289 | 107.254 | <0.0001 | 3.725 | 189.4648 | 556.243 | <0.0001 |
| Flaxseed | 1 | 2.188 | 65.402 | 59.301 | <0.0001 | 1.394 | 26.556 | 77.965 | <0.0001 |
| Water | 1 | 1.522 | 31.632 | 28.682 | 0.0003 | 2.864 | 112.039 | 328.933 | <0.0001 |
| Semolina2 | 1 | -0.448 | 2.892 | 2.623 | 0.1364 | -2.257 | 73.414 | 215.534 | <0.0001 |
| Flaxseed2 | 1 | 1.062 | 16.260 | 14.743 | 0.0033 | -1.656 | 39.520 | 116.026 | <0.0001 |
| Water2 | 1 | -1.090 | 17.107 | 15.511 | 0.0028 | -0.762 | 8.357 | 24.535 | 0.0006 |
| Semolina x Flaxseed | 1 | 0.247 | 0.489 | 0.443 | 0.5205 | 0.069 | 0.038 | 0.111 | 0.7459 |
| Semolina x Water | 1 | -0.280 | 0.625 | 0.567 | 0.4689 | -0.554 | 2.453 | 7.202 | 0.0229 |
| Flaxseed x Water | 1 | 0.308 | 0.758 | 0.687 | 0.4265 | -0.346 | 0.959 | 2.816 | 0.1243 |
| Residual | 10 | 11.029 | 3.406 | ||||||
| Lack of Fit | 5 | 4.700 | 0.743 | 0.624 | 0.957 | 0.391 | 0.8371 | ||
| Pure Error | 5 | 6.329 | 2.449 | ||||||
| R2 | 0.95888 | 0.99227 | |||||||
| Adjusted R2 | 0.92186 | 0.98531 | |||||||
Table 7: ANOVA showing the variables as a linear, quadratic, and interaction terms on gelatinization and coefficients for the prediction models.
| Source | df | Flaxseed flour based pasta | Defatted flaxseed based pasta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SS | F Value | p-value | β | SS | F Value | p-value | ||
| Model | 9 | 3.294 | 10.455 | 0.0005 | 3.0632 | 4.2421 | 0.0170 | ||
| Constant | 1 | 7.455 | 7.775 | ||||||
| Semolina | 1 | 0.214 | 0.62471 | 17.8445 | 0.0018 | 0.323 | 1.4246 | 17.7559 | 0.0018 |
| Flaxseed | 1 | -0.324 | 1.437 | 41.038 | <0.0001 | -0.093 | 0.1175 | 1.0646 | 0.040 |
| Water | 1 | 0.047 | 0.031 | 0.879 | 0.3706 | 0.103 | 0.1441 | 1.7957 | 0.2099 |
| Semolina2 | 1 | -0.173 | 0.431 | 12.306 | 0.0057 | -0.234 | 0.7880 | 9.8212 | 0.0106 |
| Flaxseed2 | 1 | 0.154 | 0.342 | 9.780 | 0.0107 | 0.164 | 0.3872 | 4.8257 | 0.0427 |
| Water2 | 1 | -0.067 | 0.064 | 1.839 | 0.2049 | 0.067 | 0.0641 | 0.7987 | 0.3925 |
| Semolina x Flaxseed | 1 | -0.185 | 0.274 | 7.821 | 0.0189 | 0.001 | 0.0000 | 0.0002 | 0.9903 |
| Semolina x Water | 1 | -0.010 | 0.001 | 0.023 | 0.8829 | -0.004 | 0.0001 | 0.0014 | 0.9709 |
| Flaxseed x Water | 1 | 0.030 | 0.007 | 0.206 | 0.6599 | 0.011 | 0.0010 | 0.0126 | 0.9128 |
| Residual | 10 | 0.350 | 0.8023 | ||||||
| Lack of Fit | 5 | 0.094 | 0.366 | 0.8526 | 0.3525 | 0.7835 | 0.6023 | ||
| Pure Error | 5 | 0.256 | 0.4499 | ||||||
| R2 | 0.95393 | 0.96267 | |||||||
| Adjusted R2 | 0.92747 | 0.93706 | |||||||
Table 8: ANOVA showing the variables as a linear, quadratic, and interaction terms on overall acceptability and coefficients for the prediction models.
Free fatty acid (%) varied from 0.83-4.49 in flaxseed flour based pasta and 0.173-0.995 in defatted flax pasta (Table 3 and 4). The higher FFA of flaxseed based pasta is due to presence of unsaturated lipids in flaxseed. Flaxseed products are stable, despite their high ALA (alphalinolenic acid) content. Storing milled flaxseed at ambient temperatures for more than 4 months did not cause noticeable changes in quality. The endogenous antioxidants in the milled flaxseed may account for this stability [27,28]. Chen et al. [28] observed that flaxseed products can tolerate heat to a certain extent. Heating at 178°C for 1.5 hours did not change the ALA content in the whole flaxseed. ALA decreased from 55.1 to 51.3% in ground flaxseed under the same conditions, and the ALA content dropped to 51.7% in lipid extracts under the same conditions. The magnitude of p values revealed that the linear and quadratic terms of flaxseed concentration had significant effect (p<0.05) on FFA (Table 6). Semolina contains less than 4% lipids, out of which more than 50% are saturated; as a result, the effect of semolina content on FFA is insignificant. The model F-value was 27.459 and 82.311 for flaxseed and defatted flaxseed based pasta, which implies that both models are significant. FFA (%) increased with amount of flaxseed in both the flaxseed and defatted flaxseed pasta, as shown in figure 1a and b.
Gelatinization is of utmost importance in food modifications, such as those that take place in cooking, baking, drying and extruded foods. The honeycomb-like structure of cooked pasta is mainly due to the coagulated protein embedded in the gelatinized starch. Determination of pasta cooking quality was more dependent on a continuous protein network than the physicochemical properties of the gelatinized starch. In the absence of coagulated protein, “starch pasta” strands fractured into small pieces and did not swell [29]. The gelatinization (%) varied from 48.172- 73.714 in flaxseed flour based pasta and 57.4-89.66 in defatted flax pasta (Table 3 and 4). Lower gelatinization in flaxseed based pasta may be attributed to high lipid content as lipid binds to starch granules and stabilizes the granular structure. Lauro et al. [30] and Elaisson et al. [31] observed decreased gelatinization of starch with increasing amounts of lipids. Gelatinization increased with amount of semolina, defatted flaxseed and water. The magnitude of p values from table 7 revealed that all linear and quadratic terms of process variables had significant effect (p<0.05) on gelatinization of both flaxseed and defatted flaxseed based pasta. Semolina content had most significant effect on gelatinization, followed by water content and flaxseed content (Table 7). Further, interaction terms had no significant effect (p>0.05) on gelatinization. The model F-value was 257.907 and 142.621 for flaxseed and defatted flaxseed based pasta, which implies that both models are significant. The relative magnitude of β values indicated the maximum positive effect of amount of semolina (β=2.943 and 3.725), followed by amount of water (β=2.188 and 2.864) and amount of flaxseed (β=1.522 and 1.394), on gelatinization for flaxseed and defatted flaxseed based pasta (Table 7). The quadratic and interaction terms of all the process parameters had least effect on gelatinization, as compared to the linear terms of process variables. The effect of process parameters on gelatinization is shown in figure 2a and b.
Effect of various process parameters on Overall Acceptability (OAA) is indicated in figure 3a and b. The overall acceptability of cooked pasta varied from 6.8 to 8.4, with the change in process parameters (Table 3 and 4). Semolina content had most significant effect on overall acceptability of flaxseed based and defatted flaxseed based cooked pasta. OAA did not increase with amount of flaxseed, which may be due to brown color of flaxseed and fishy odour of omega-3 fatty acids, which increased with amount of flaxseed (Figure 3a and b) and negative β value was observed (Table 8). OAA decreased with amount of semolina and increase in amount of flaxseed flour. The results are in agreement with the studies of Ogunronbi et al. [32] on flax based bread, and Khouryieh and Aramouni [33] on flax based cookies. Water content had non significant effect on OAA of both flaxseed and defatted flaxseed based pasta. All other process variables have significant effect (p<0.05) at linear and quadratic levels on OAA. The model F-value was 10.455 and 4.241 for flaxseed and defatted flaxseed based pasta, which implies that both models are significant (Table 8).
The color of the pasta plays a major role in consumers’ perception and acceptability of the product. The color parameter values, (L* and a*), for flaxseed and defatted flaxseed based pasta are presented in table 3 and 4, respectively. The values of color parameter (b*) in pasta were found to be low, so were not included in optimization studies. The values of color parameters (L*) of defatted flaxseed pasta were higher than that of flaxseed based pasta, is possibly due to removal of oil. The linear and interaction terms of flaxseed or defatted flaxseed had a significant effect on color of pasta (Table 9a and 9b). All other process parameters except quadratic term of semolina had a non significant effect on color of pasta. The model F-value and non significant lack of fit presented in table 9a and 9b for flaxseed and defatted flaxseed based pasta, implies that both models are significant. The observed results are in agreement with the studies of Khouryieh and Aramouni [33], on flax based cookies. The effect of process variables on color parameters (L* and a*) is shown in figure 4a-4d.
| Source | df | Flaxseed flour based pasta | Defatted flaxseed based pasta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SS | F Value | p-value | β | SS | F Value | p-value | ||
| Model | 9 | 348.465 | 44.953 | <0.0001 | 346.244 | 38.568 | <0.0001 | ||
| Constant | 1 | 66.5659 | 74.4488 | ||||||
| Semolina | 1 | 0.241 | 0.79347 | 0.92123 | 0.3598 | 0.241 | 0.79347 | 0.79546 | 0.3934 |
| Flaxseed | 1 | 4.643 | 294.391 | 341.795 | <0.0001 | 4.730 | 305.504 | 306.272 | <0.0001 |
| Water | 1 | -0.350 | 1.67216 | 1.94142 | 0.1937 | -0.350 | 1.672 | 1.676 | 0.2245 |
| Semolina2 | 1 | -1.280 | 23.626 | 27.430 | 0.0004 | -0.558 | 4.488 | 4.500 | 0.0599 |
| Flaxseed2 | 1 | 1.092 | 17.172 | 19.938 | 0.0012 | 1.336 | 25.712 | 25.777 | 0.0005 |
| Water2 | 1 | 0.371 | 1.986 | 2.306 | 0.1598 | 0.386 | 2.152 | 2.158 | 0.1726 |
| Semolina x Flaxseed | 1 | 0.028 | 0.006 | 0.007 | 0.9331 | 0.028 | 0.006 | 0.006 | 0.9378 |
| Semolina x Water | 1 | 0.586 | 2.747 | 3.190 | 0.1044 | 0.586 | 2.747 | 2.754 | 0.128 |
| Flaxseed x Water | 1 | -0.418 | 1.398 | 1.623 | 0.2315 | -0.418 | 1.398 | 1.401 | 0.2639 |
| Residual | 10 | 8.613 | 9.975 | ||||||
| Lack of Fit | 5 | 3.409 | 0.655 | 0.6731 | 4.054 | 0.685 | 0.6561 | ||
| Pure Error | 5 | 5.204 | 5.921 | ||||||
| R2 | 0.97588 | 0.972 | |||||||
| Adjusted R2 | 0.95417 | 0.9468 | |||||||
Table 9a: ANOVA showing the variables as a linear, quadratic, and interaction terms on color (L*) and coefficients for the prediction models.
| Source | df | Flaxseed flour based pasta | Defatted flaxseed based pasta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SS | F Value | p-value | β | SS | F Value | p-value | ||
| Model | 9 | 2.432 | 22.778 | <0.0001 | 1.964 | 19.322 | <0.0001 | ||
| Constant | 1 | 3.473 | 3.185 | ||||||
| Semolina | 1 | -0.131 | 0.233 | 19.606 | 0.0013 | -0.099 | 0.1339 | 11.867 | 0.0063 |
| Flaxseed | 1 | 0.364 | 1.805 | 152.11 | <0.0001 | 0.338 | 1.5577 | 137.961 | <0.0001 |
| Water | 1 | 0.006 | 0.001 | 0.045 | 0.836 | 0.014 | 0.0025 | 0.22326 | 0.6467 |
| Semolina2 | 1 | -0.071 | 0.072 | 6.097 | 0.0331 | -0.050 | 0.036 | 3.217 | 0.1031 |
| Flaxseed2 | 1 | -0.152 | 0.332 | 27.994 | 0.0004 | -0.122 | 0.213 | 18.879 | 0.0015 |
| Water2 | 1 | -0.033 | 0.016 | 1.353 | 0.2718 | -0.047 | 0.032 | 2.864 | 0.1214 |
| Semolina x Flaxseed | 1 | 0.003 | 0.000 | 0.008 | 0.9294 | -0.009 | 0.001 | 0.057 | 0.8155 |
| Semolina x Water | 1 | -0.023 | 0.004 | 0.341 | 0.572 | -0.010 | 0.001 | 0.071 | 0.7955 |
| Flaxseed x Water | 1 | -0.036 | 0.010 | 0.862 | 0.3751 | -0.048 | 0.019 | 1.649 | 0.228 |
| Residual | 10 | 0.119 | 0.113 | ||||||
| Lack of Fit | 5 | 0.050 | 0.725 | 0.6336 | 0.044 | 0.642 | 0.6809 | ||
| Pure Error | 5 | 0.069 | 0.069 | ||||||
| R2 | 0.95349 | 0.96562 | |||||||
| Adjusted R2 | 0.9363 | 0.93668 | |||||||
Table 9b: ANOVA showing the variables as a linear, quadratic, and interaction terms on color (a*) and coefficients for the prediction models.
Optimization of process variables for the development of flaxseed and defatted flaxseed based pasta
Graphical multi-response optimization technique was adopted to determine the optimum conditions for the osmo-convective dehydration of ginger cubes. The contour plots for all responses were superimposed, and regions that best satisfy all the constraints were selected as optimum conditions. The main criterion for constraints optimization was minimum possible %FFA, and color parameter (a*) and maximum possible %gelatinization, color parameter (L*) and OAA. These constraints resulted in “feasible zone” of the optimum solutions (shaded area in the superimposed contour plots).
To optimize the process variables for the development of flaxseed and defatted flaxseed based pasta by numerical optimization technique, equal importance of ‘3’ was given to all three process parameters (viz. semolina, flaxseed or defatted flaxseed and water content) and 5 responses (viz. %FFA, %gelatinization, OAA, L* and a*). For the preparation of a functional food having properties of flaxseed, while optimizing the flaxseed content was selected in range of 15-30% because it is beneficial from the nutritional and therapeutic point of view. The optimum values of process variable for semolina, flaxseed and water content are represented in table 10a and 10b. The optimum processing conditions were experimentally verified and proven to be adequately reproducible with ± 0.1% deviation.
| Responses | Optimum conditions | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Criteria | Predicted value | Observed value | Desirability | Semolina (g) | Flaxseed (g) | Water* |
| %FFA | Minimum | 1.83 | 1.95 | 0.652 | 90 | 15 | 20 |
| %Gelatinization | Maximum | 64.75 | 65.91 | ||||
| OAA | Maximum | 7.74 | 7.6 | ||||
| Color (L*) | Maximum | 64.28 | 65.72 | ||||
| Color (a*) | Minimum | 3.02 | 3.07 | ||||
* Water (ml)=% of Semolina (g)+Flaxseed (g)
Table 10a: Optimum solution for various process variables for development of flaxseed based pasta.
| Responses | Optimum conditions | ||||||
|---|---|---|---|---|---|---|---|
| Parameters | Criteria | Predicted value | Observed value | Desirability | Semolina (g) | Flaxseed (g) | Water* |
| %FFA | Minimum | 0.37 | 0.38 | 0.789 | 87.79 | 15 | 30 |
| %Gelatinization | Maximum | 88.29 | 87.82 | ||||
| OAA | Maximum | 8.13 | 8.07 | ||||
| Color (L*) | Maximum | 72.96 | 73.09 | ||||
| Color (a*) | Minimum | 2.86 | 2.78 | ||||
* Water (ml)=% of Semolina (g)+Defatted Flaxseed (g)
Table 10b: Optimum solution for various process variables for development of defatted flaxseed based pasta.
Response surface methodology was successfully used in optimizing process parameters for the development of flaxseed and defatted flaxseed based pasta. Graphical technique was applied to locate optimum operating conditions, which were experimentally verified and found to be adequately reproducible. Optimum solutions using numerical optimization obtained were: semolina 90 g, flaxseed 15 g and water 20 (% of amount of semolina and flaxseed) for development of flaxseed based pasta, and semolina 87.79 g, flaxseed 15 g and water 30 (% of amount of semolina and flaxseed) for development of defatted flaxseed based pasta, to get maximum possible gelatinization, color parameter (L*), overall acceptability, minimum free fatty acid and color parameter (a*). Thus, flaxseed and defatted flaxseed based pasta was developed, having functional properties of flaxseed.