Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2016) Volume 6, Issue 1
Fresh, unrefined argan (AR), chia (CH), rosa mosqueta (RM) and olive (OL) oils were evaluated for thermal stability with dielectric spectroscopy, differential scanning calorimetry, peroxide values (PV) and free fatty acids (FFAs). The dielectric constant ( '), dielectric loss ( ''), and melting temperature (Tm) of the oils correlated with degree of unsaturation, with the highest static dielectric constant (εs') for CH oil (3.311), the most unsaturated. AR and CH oils showed the highest electrical conductivities at 500 Hz (505, 230 pS/m, respectively), and CH and RM oils the lowest Tm (-41.0, -36.6°C, respectively). All oils stored at 65°C for 432 hr showed minor changes in εs'. After 180°C for 28 hr, εs' and ε'' increased from 5% to 7%. Initial PVs of these oils were <3.7 mmol O2/kg, and for highly unsaturated CH oil this increased to 221 mmol O2/kg after 65°C for 432 hr. At 180°C, the FFAs increased in all oils, except AR oil. Oxidation onset temperature of the oils correlated with PVs after 65°C for 432 hr (r=-0.91) and with FFAs and εs' after 180°C for 28 h (r=-0.81, -0.83, respectively).
Keywords: Unrefined oil; Argan, Chia, Rosa mosqueta, Olive oils; Oxidative stability; Dielectric constant; Differential scanning calorimetry; Peroxide value
Oxidative stability is one of the most important indicators in the assessment of the quality and shelf-life of oils, and it can be defined as the resistance of the oil to oxidation. Lipid oxidation is a complex process that is initiated by free radical reactions that involve unsaturated fatty acids (UFAs). This process can be accelerated at elevated temperatures (i.e., thermal oxidation) with increases in free fatty acids (FFAs) and polar compounds [1,2].
A number of analytical methods have been developed to estimate the deterioration of edible oils. Among this dielectric spectroscopy has emerged as a simple, rapid and non-destructive measuring technique that can provide information about the dielectric responses of materials to electromagnetic fields. The dielectric properties of most materials depend on the frequency of the applied alternating electric field, and on the temperature, moisture content, density, composition, and structure of the material [3]. These have been used extensively for the analysis and monitoring of the quality of various foods [4,5]. Differential scanning calorimetry (DSC) is another method that can be used to determine the oxidative stability of oils and fats [6,7]. The oxidation and thermal-oxidative decomposition of oils and fats are exothermal reactions, and DSC thermo grams reflect these changes. DSC is also advantageous over other methods because it is more precise and sensitive, and it requires smaller amounts of sample and produces results rapidly [8].
In the present study, the oxidative stabilities at elevated temperatures of three unrefined oils were determined: argan (AR), chia (CH), and rosa mosqueta (RM) oils. Furthermore, these were compared to the oxidative stability of a well-characterised unrefined olive (OL) oil and to two refined edible oils, rapeseed (RS) and sunflower (SF) oils.
AR oil is obtained from the roasted kernels of the nuts of the argan tree (Argania spinosa L. Skeels). The consumption of AR oil has recently increased in the European, North American, and Japanese oil markets [9]. Two oilseeds from Argentina were also used: from chia (Salvia hispanica L) and Rosa mosqueta (Rosa rubiginosa L.). CH oil is an interesting source of polyunsaturated FAs (PUFAs) [10]. However, the RM oil FA composition is not well known. This is obtained from the seeds from a wild shrub that grows in the Andean region and in some specific areas of central Europe. OL oil is rich in monosaturated FAs and is very popular as part of the Mediterranean diet.
For these investigated oils, The FA composition and melting temperature (Tm) were initially determined. Also, the oil stabilities were determined using DSC, including the onset-of-oxidation temperature (OOT), and the dielectric constant (ε'), dielectric loss (ε''), and electrical conductivity (σ) determination during thermal treatments at two different temperatures (i.e., 65, 180°C). The standard oil degradation parameters of peroxide value (PV) and FFAs were also determined.
Materials
For the unrefined oils, AR oil originated from Tunisia, RM and CH oils from Argentina, and OL oil from Slovenia. These were compared with refined, blanched, deodorised oils, as RS and SF, from Slovenia. All of the other reagents and solvents were of analytical purity.
Thermal treatment
Oil samples (ca. 20 g) were put into open glass flasks and heated in an oven (Memmert vacuum oven VO 400; GmbH & Co., Schwabach, Germany) for accelerated storage at 65°C for up to 432 hr (18 days), or heated under simulated frying conditions at 180°C for 28 hr. After heating, the samples were cooled and stored in screw-capped amber bottles at 4°C for further analyses.
Determination of fatty acid composition in fresh oils
The FA compositions of all of the fresh oils were determined using gas chromatography. The FA methyl esters (FAMEs) were prepared by trans-esterification using the boron trifluoride method, according to AOAC Official Method 969.33 [11]. The analyses were performed on a gas chromatograph (Agilent Technologies 6890N; Agilent Technologies, Palo Alto, CA, USA) equipped with a fused silica capillary column (Supelco SPB PUFA; 30 m × 0.25 mm × 0.2 μm). The carrier gas was helium at a flow rate of 1 mL/min. Heptadecanoic acid (H3500; Sigma) was used as the internal standard, with 1.5 mg per sample. The column temperature was programmed to 210°C. Injector (split 1:100) and flame-ionisation detector temperatures were set at 250°C and 260°C, respectively. The samples were injected in triplicate at a pressure of 31.6 psi, with an injection volume of 1 μL. The FAs were identified by comparisons of their retention times with those of the FAME standards mixture (Supelco, Bellefonte, Pennsylvania, USA). Quantitative data were obtained from the electronic integration of the flame-ionisation detector peak areas. The data are given as weight percentages of the determined FAs.
Dielectric spectroscopy
The dielectric parameters of the oils (as both fresh and thermally treated) were determined using a liquid test fixture (16452A) and a precision LCR meter (E4980A) (both from Agilent Technologies, Hyogo, Japan), from the capacitance (Cp), and the equivalent resistance (Rp) measurements. The dielectric parameters were measured at 25 uniformly distributed frequencies in the range from 500 Hz to 2 MHz at 20.0°C ± 0.1°C. The temperature control was performed using a high-precision water bath (Fluke 7320) and an external thermometer system (Fluke 5627A/1502A; Hart Scientific, Everett, WA, USA). Before each determination, the samples were filtered in the presence of ca. 2 g of anhydrous sodium sulphate in a vacuum oven (MIR-154; Sanyo, Japan) at 100 mbar and 25°C, to remove all traces of water and to allow degassing. The ε’, εs’’ and of the oil samples were calculated using the following equations:
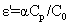 (1)
(1)
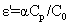 (2)
(2)
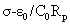 (3)
(3)
Where Cp and C0 are the capacitances of the model lipid and of air, respectively, α is the correction coefficient according to the calculations (Agilent technologies; [12]), Rp is the equivalent parallel resistance, ω is the angular frequency (2πf) and 0 is the permittivity of the vacuum (8.854 pF/m).
Differential scanning calorimetry
The Tm and OOT of the fresh oils and the OOT of the oils treated at 65°C were determined using DSC. A differential scanning calorimeter (DSC 822e; Mettler Toledo AG, Schwerzenbach, Switzerland) and the STARe Thermal Analysis System version 3.1 software (Mettler Toledo AG, Schwerzenbach, Switzerland) were used. The instrument was calibrated using standard compounds (i.e., indium, zinc) of defined melting temperatures and heat of melting. All of the measurements were made in duplicate, with 5.0 ± 0.5 mg oil weighed into an open aluminium pan, and an empty one used as reference. The samples were initially held at ambient temperature (25°C) for 2 min. This step was followed by heating to 220°C at a heating rate of 10°C/min under air. The OOT was taken at the intersection of the extrapolated baseline and the tangent line (i.e., leading edge) of the exothermal peak. The oils were also heated from -80°C to 20°C under nitrogen to obtain the Tm.
Peroxide value
The PVs of the fresh and thermally treated oils were determined using the iodometric method, according to AOAC Official Method 965.33 [11]. The determinations were carried out in triplicate, and PV is expressed as mmol O2/kg oil.
Free fatty acids
The FFA content expressed as percentages of oleic acid were determined for the fresh oils and for those treated at 180°C, according to AOCS Official Method Ca 5a-40 [11]. The determinations were carried out in triplicate.
Characteristics of the fresh oils
Fatty acid composition: The unrefined fresh AR, CH, RM and OL oils were analysed for their main FAs. These data are given in Table 1, where the ratios of unsaturated to saturated fatty acids (UFA/SFA) are given along with the iodine values of the oils. The iodine values of the oils were calculated (as g I2/100g of oil) from their FA composition, to provide information on the degree of unsaturation of the oils. The FAs of the two refined edible oils (i.e., SF, RS oils) are also given in Table 1 for comparison. It should be noted that both the AR and OL oils had higher contents of 16:0 FA than the other oils analysed, and OL oil had the highest content of 18:1 FA. CH oil was especially rich in 18:3 FA, and had the highest overall PUFA content (76.1%). RM oil had the highest UFA/SFA ratio (14.2), and as with SF oil, it was high with 18:2 FA.
| Oil | FA (% total, by weight) | UFA/SFA | IV (g I2/100g) | εs' | σ (pS/m) | Tm (°C) | PV (mmol O2/kg) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16:00 | 18:00 | 18:01 | 18:02 | 18:03 | |||||||
| AR | 12.73 ± 0.04 | 5.7 ± 0.1 | 44.1 ± 0.3 | 36.7 ± 0.4 | 0.79 ± 0.03 | 4.4 | 104 | 3.15 | 505 ± 3 | -12.6 ± 0.5 | 2.45 ± 0.04 |
| CH | 7.1 ± 0.2 | 2.8 ± 0.2 | 6.0 ± 0.3 | 20.9 ± 0.3 | 63.3 ± 0.4 | 8.8 | 206 | 3.31 | 230 ± 6 | -41.0 ± 0.5 | 2.03 ± 0.02 |
| RM | 4.35 ± 0.02 | 2.25 ± 0.02 | 17.3 ± 0.09 | 49.4 ± 0.08 | 26.7 ± 0.2 | 14.2 | 170 | 3.25 | 21 ± 2 | -36.6 ± 0.5 | 1.97 ± 0.11 |
| OL | 11.3 ± 0.2 | 2.8 ± 0.2 | 76.3 ± 0.3 | 8.8 ± 0.3 | 0.77 ± 0.05 | 6.2 | 83 | 3.16 | 14 ± 1 | -3.7 ± 0.5 | 3.66 ± 0.19 |
| RS | 5.92 ± 0.02 | 2.30 ± 0.03 | 55.91 ± 0.09 | 29.04 ± 0.06 | 6.83 ± 0.04 | 11.3 | 116 | 3.17 | 14 ± 3 | -18.4 ± 0.5 | 0.76 ± 0.09 |
| SF | 6.48 ± 0.01 | 3.37 ± 0.01 | 30.04 ± 0.03 | 59.75 ± 0.05 | 0.36 ± 0.01 | 9.2 | 130 | 3.19 | 2 ± 1 | -25.0 ± 0.5 | 1.02 ± 0.12 |
Table 1: Main analytical characteristics of the fresh oils used in this study, AR, argan oil; CH, chia oil; RM, rosa mosqueta oil; OL, olive oil; RS, rape seed oil; SF, sunflower oil; FA, fatty acids (mean ± standard error; n=3); UFA/SFA, ratio of unsaturated fatty acids to saturated fatty acids; IV, iodine value; ε's , static dielectric constant (mean of frequencies from 500 to 1000 Hz; n=10; standard error <3×10-4); σ electrical conductivity at 500 Hz (mean ± standard error; n=2); Tm, melting temperature (mean ± standard error; n=2); PV, peroxide value (mean ± standard error; n=3).
Dielectric parameters: The ε’ was calculated from CP according to Equation 1, and the data obtained from these unrefined and refined oils showed the same frequency dependence for ε’ (Figure 1A). In the frequency range from 500 Hz to 1 MHz, the dielectric spectra of the tested oils indicated a general plateau. At these frequencies, the orientation of the molecule is in equilibrium with the electric field, and ’ indicated a constant and maximum value, known as the static dielectric constant (ε s ’ ).. This constant was calculated as its mean in the frequency range from 500 Hz to 1000 Hz, and is given in Table 1. In the frequency range from 1 MHz to 2 MHz, ’ diminished in agreement with the Debye equation, which shows that ’ decreases from ’ to ∞’ as the frequency increases.
From the Rp data, ε’’ and σ were calculated from Equations (2) and (3), respectively, and their dependence on frequency is shown in Figure 1B, C, respectively. The ε’’ of the investigated oils was higher at low frequencies compared to medium frequencies (5-100 kHz), where ε’’ showed a long minimum, which then increased to the highest values of ε’’ at 2 MHz (Figure 1B). The σ of both the unrefined and refined oils were low (Table 1), and increased to 2 to 3 × 106 pS/m with increasing frequency (Figure 1C). All of the oils except AR and CH oils showed similar characteristics. At low frequencies, AR and CH oils had much higher values of both of these ε’’ and σ parameters. Indeed, it is known from the literature that the ionic contributions to ε’’ and σ predominate at frequencies <20 kHz, while the dipole effect and its conductive equivalent predominate at frequencies >20 kHz [13]. To describe the differences among the investigated oils, the representative values of s’, ε’’ (determined at 2 MHz) and σ (determined at 500 Hz) were selected.
Figure 2A illustrates the linear increase for εs’ and Figure 2B, the linear decrease for ε’’ versus the iodine value. The Pearson’s correlation coefficients are R=0.975 for εs’ and -0.988 for ’’, even though the error of determination of ’’ is ca. 5%. Here, εs’ of CH oil, which is rich in 18:3 FAs, was surprisingly high. To date, high s’ values have been found only for triacylglycerols with shorter SFAs [5,13]. Also here, the εs’ increases for AR, OL and RS oils, as correlated with the increasing UFA/SFA ratios in the oils, and differences between the oleic and linoleic types of these oils have been confirmed [5].
The data for σ (determined at 500 Hz) for the investigated fresh oils are given in Table 1. For the unrefined oils, the sequence followed the order of OL < RM < CH < AR from 14 pS/m to 505 pS/m. These data are comparable to those determined in different studies [14,15]. This relatively large difference in σ across these studied oils can be ascribed to their mineral and phospholipid contents [16], and does not depend on the FA composition. This explanation can be applied to AR and CH oils, where the high values of σ are connected to the higher mineral ion content determined for unrefined vegetable oils extracted from seeds. On the other hand, lower σ were determined for OL oil and the refined oils, which had much lower concentrations of ions [17].
Differential scanning calorimetry: These oils were also analyzed by DSC. The thermo gram profiles were similar for all of the oils, and showed two well-distinguished events when heated from -80°C to 20°C. The first is an exothermic peak that can be attributed to the transition/rearrangement of triacylglycerol polymorphic crystals into more stable forms. The second endothermic peak occurred at higher temperatures, and this was characterized by multiple overlapping contributions [7]. The Tm linearly decreased when the degree of unsaturation of the oils (expressed as the iodine value) increased (R=-0.976) (Figure 2C). This has also been observed for other vegetable oils [6]. When the Tm was related to the UFA/SFA content in these tested oils, a correlation with R=-0.635 was obtained.
Oxidative stability of the oils
The oxidative stability of the oils after exposure to different thermal treatments for different times (t) were evaluated according to the PV, FFAs, dielectric parameters, and OOT.
Peroxide value: The primary products of lipid oxidation are hydroperoxides, which are generally referred to as peroxides.
Therefore, the determination of PV can be used as an index of the firststep lipid oxidation.
At 65°C, steep increases in PVs were observed after 336 h of treatment, while at 180°C, the rate of peroxide formation was almost constant over the period studied (28 hr) (Figure 3). The initial PVs of the oils before the thermal treatment were from 0.8 to 3.7 mmol O2/kg (Table 1). After 432 hr at 65 °C, the PVs varied from 10 to 221 mmol O2/kg, while in the samples treated at 180°C, after 28 hr the PVs increased only up to 9 mmol O2/kg oil, due to the continuous degradation of peroxides provoked by this temperature. The investigated oils from the lowest to the highest PVs indicate the stability of these oils at 65°C, according to the following sequence: OL > SF > AR > RM > RS > CH.
At 65°C, PVs showed exponential dependence with t, expressed as: PV=PV0ekPV were k PV is a kinetic constant (Figure 3A). The k PV obtained by exponential regression analysis was in the range between 0.003/hr and 0.012/hr, according to the following sequence: OL < AR ≅ SF < RM < CH < RS. However, at 180°C, the PVs increased linearly with t (corresponding to zero-order kinetics): PV=PV0 + kPVt for all of the investigated oils, with the exception of OL oil, where PV increased with t as a power function (Figure 3B). The oils with linear PV dependence on t show the following sequence according to their kPVt: AR < SF < CH < RS. The hydroperoxide concentrations determined are the result of the hydroperoxides accumulated and their decomposition in the samples during the treatments at 65°C and 180°C. In the samples incubated at 180°C, the decomposition of hydroperoxides was accelerated, giving rise to reaction products such as aldehydes, ketones, alcohols, and shorter FAs, among others [1,18]. For OL oil, the steep increase in PV in the first 5 hr of thermal treatment indicates rapid first-step oxidation, while with prolonged thermal treatment, the decay of hydroperoxides due to their transformation to other secondary oxidation products predominated.
Dielectric parameters: The shape of the dielectric spectra of the thermally treated oils at 65°C and 180°C remained almost the same as for the fresh oils. Only slight reductions of ε' and rising of ε’’ at frequencies close to 2 MHz, and some differences in conductivity at 500 Hz, were noted.
During thermal treatment, εs’ determined from 500 Hz to 1000 Hz for all of the oils treated at 65°C showed minor changes. At 180°C, the time dependence of the dielectric parameters εs’ and ε’’ (at 2 MHz) followed power functions (Figure 4). After an initial steep increase, with prolonged thermal treatment the dielectric parameters increased more slowly. This can be ascribed to the lower mobility of the molecules caused by the increase in the viscosity and density of the thermally treated and deteriorated oils [19]. After 180°C for 28 hr, εs’ increased from around 5% to 7% for all of the oils. The values obtained are comparable to the data of ε’ determined using Food Oil sensors [20], and indicate an increase in the polar compounds formed as a product of the hydrolysis, oxidation and polymerization processes that can occur at elevated temperatures.
The conductivity of all of these oils except for AR oil increased during the first 5 h of incubation at 180 °C, from the initial values presented in Table 1 to 630, 38, 22, and 24 pS/m for CH, OL, RS and SF, respectively, while σ of AR oil decreased from 505 pS/m to 400 pS/m. With the prolonging time of incubation, σ remained unchanged for all of the oils studied.
Differential scanning calorimetry: Due to the sensitivity and short determination time the DSC method was also used for evaluation of the oxidative stability of these investigated oils. OOT was measured for fresh oils, and for comparison, for thermally treated oils (after 65°C for 336 h) by heating oil samples to 220°C. The oils treated at 180°C were completely oxidised and the determination of OOT was not possible. Flat curves were observed under a nitrogen atmosphere, while exothermic oxidation curves were obtained running the experiment in air. The OOT values of fresh oils from 147°C to 194°C were according to the series: CH < RM < SF < RS < AR < OL. When OOT was correlated to the iodine value, good correlation was obtained too (R=-0.989). The OOT of the oils thermally treated at 65°C decreased to values ranging from 106°C to 177°C. The investigated oils followed the sequence CH < RS < AR < RM < SF < OL. The observed decrease in OOT most probably occurred due to the naturally present antioxidants being destroyed [21,22], and due to the presence of oxidative reactive compounds generated during the thermal treatment. Casal et al. [20] reported that in OL oil incubated at 170°C, all of the vitamin E was lost after 3 hr to 6 hr, while total phenolics were also completely degraded after 6 hr in almost all samples. As already observed for PV, OL oil showed the highest resistance to oxidation. The higher content of naturally present antioxidants in OL oil explains the observed results, with its oxidative stability that is dependent on its FA content, and particularly of monounsaturated FAs, as these have low relative reaction rates with oxygen [2,23].
Free fatty acids: FFA content of the fresh oils was between 0.1% for RS and 0.8% for AR with the exception of CH with 1.9% FFAs. The limit of FFAs as established by European regulations for unrefined oils is 2%. Thermal treatment of OL, RS and SF oils at 180°C after 28 hr resulted in increased FFAs by 2-fold to 3-fold. There were almost no changes in FFAs for AR oil, while 22% less FFAs was determined in CH oil, with the correlation coefficient with t of R=-0.971. Elevated temperatures provoke de-esterification of triacylglycerols, and consequently the formation of FFAs. On the other hand, FFAs are susceptible to degradation to aldehydes, alcohols and some other products, which can give a rancid-like aroma and flavour [18]. Highly unsaturated FAs in oils are particularly vulnerable to oxidation, and their oxidation ratio gives oleic: linoleic: linolenic acid 1:25:50 [24]. CH oil is one such oil, due to its high content of α-linolenic acid (18:3). The decrease in FFAs in CH oil can therefore be attributed to the prompt oxidation and subsequent degradation of this FA.
Correlation analyses
The results of εs’, OOTs, PVs and FFAs were compared to search for correlations between these data and the oil compositions (Figures 5 and 6). Figure 5 shows the dependence between εs’ and PV for the unrefined oils and the RS and SF oils incubated at 65°C and at 180°C. The thermal treatment of the oils at 65°C for 432 hr resulted in a greater increase in PV and a less pronounced increase in εs’ than in the oils treated at 180°C for 28 hr. At elevated temperatures (180°C), the products formed after hydrolysis, oxidation and/or polymerisation had higher polarity than the hydroperoxides. Therefore, they probably contributed to the more pronounced increase in εs’ than at 65°C, which is in agreement with the previous discussion related to the kinetics of hydroperoxide decomposition/accumulation.
Figure 6 shows that the data for PV as the primary oxidation products in the oils treated at 65°C for 432 hr, and FFA and εs’ as the secondary oxidation products in the oils heated to 180°C for 28 hr, are correlated with OOT of the fresh oils. The correlation of PV versus OOT is inversely proportional (R=-0.91), which means that oil with lower OOT is oxidised easily (Figure 6A). After incubation at 180°C, both FFAs (Figure 6B) and εs’ (Figure 6C) versus OOT for the fresh oils showed a similar tendency; i.e., FFAs versus OOT (R=-0.81) and εs’ versus OOT (R=-0.83). The exceptions are AR oil, which had a surprisingly low εs’, and OL oil, with higher εs’. This might indicate that the thermal treatment of the AR oil caused different changes than for the OL oil. In the AR oil, there was higher content of hydrolysis products, as FFAs, while in the OL oil; there was higher content of polar compound, as εs’. These data indicate again the complexity of the changes in such oils at elevated temperatures.
The oxidative stability of AR, CH and RM oils has been investigated less than their FA composition; furthermore, the dielectric properties of the selected oils (and especially when thermally treated) can rarely be found in the literature. The data from the present study show that the susceptibility of the unrefined oils to oxidation is related to their UFA content. In general, the parameters determined for the oxidative stability show that among the unrefined oils, except for OL, AR was the most stable. The dielectric measurements were highly sensitive for the evaluation of oil degradation during incubations at 180°C. At a moderate temperature (65°C), the stability of these oils (determined as PV) was better detected by changes in OOT. PVs of the oils treated at 65°C were higher than PVs of the oils treated at 180°C, which suggests that at elevated temperatures accelerated decomposition of hydroperoxides and complex changes occurred. OOT of the unrefined oils correlated well with FFAs and to degree of unsaturation, but not to the dielectric constant. OOT determination has been shown once again to be a rapid and sensitive tool to follow the progress of oxidation in oils, and thus for their shelf-life evaluation, even for unrefined oils. However, we believe that to obtain the relevant information on oxidative stability for unrefined oils, different approaches are necessary.
The authors acknowledge the financial support of CONICET (PIP 100846, 100468), UBA (Project UBACyT X-024), ANPCYT (PICT 2013 1331) and MINCYT-MHEST (SLO/08/10 2009-2011), and of the Slovenian Research Agency (Program P4-0121). MFM and MPB are members of CONICET.