Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Review Article - (2025)Volume 15, Issue 3
Milk is containing immunoglobulins, lactoferrin, lysozyme, lactoperoxidase, peptidoglycan recognition protein, vitamins C and oligosaccharides which are against microbial infections. Lactoferrin is one glycoprotein detected in milk of livestock such as cow, buffalo, and camel, as camel milk containing highest amount compared to the milk from other livestock species. Activation, proliferation, and regulation of the phagocytic action of immune cells are facilitated by the lactoferrin. The antiviral actions of lactoferrin are against both DNA and RNA viruses such as hepatitis, herpes simplex viruses, HIV, rotavirus, and respiratory viruses by binding viral particles, inhibit viral adhesion, and entry into target cells. Also, lactoferrin may directly interact with viral receptors such as heparan sulfate on the cell surfaces and prevent the virus attachment and infection. The boosting host immune system by nutritional supplements such as lactoferrin may be effective against viruses’ entry and infection into the host cells. Milk lactoferrin as powder or tablets may be a novel promising candidate and preventative treatment for more severe cases of viral infections. However, it needs more studies on dosage to verify its efficacy on prevention and treatment.
Milk; Immunoglobulins; Vitamins C; Oligosaccharides; Lactoferrin; Viral infections
Lactoferrin as innatiory defense agent against mucosal infections on the cell surfaces; inhibits viral adhesion and entry into host cells, blocks the interaction of viruses with heparan sulfate receptors, binds host cells and or viral particles and prevents nuclear localization [1]. The antiviral activity of lactoferrin is against DNA and RNA viruses, enveloped and naked viruses such as rotavirus, respiratory syncytial virus, herpes simplex viruses and HIV [2]. The cationic nature of lactoferrin may be responsible for the binding capacity to viral surface [3]. Lactoferrin inhibits viral invasion to the cells and avoiding the infection [4].
The inhibiting action of lactoferrin on viruses done in the early phase of viral infection more than prevention of virus replication in the infected cell [5]. Also, the antiviral functions of lactoferrin through avoiding the virus’s entry more than stimulation of the immune cells [6]. According to the studies, bovine and human lactoferrins no had significant difference in the antiviral effects, and they prevent virus entry into the host cell by blocking receptors or connecting to viruses in the early phase of the infection [7]. However, some studies proved that bovine milk lactoferrin has greater antiviral effects than human milk lactoferrin [8]. lactoferrin is the most active anti-viral protein in the milk than b-lactoglobulin and a-lactalbumin [9].
Researchers reported lactoferrin inhibits human respiratory syncytial virus more than human milk lactoferrin. Also, human lactoferrin neutralizes simplex viruse-1 and prevent the intracellularly spread of viruses. Strong activity of lactoferrin against Human Immunodeficiency Virus (HIV) was due to inhibition of viral replication in the host cells [10]. Both apo and holo-lactoferrin have antiviral activity against HIV, but apo form may show more inhibitory effects than holo-lactoferrin. Lactoferrin blocks the internalization of some viruses into the host cell, such as poliovirus type 1, herpes simplex virus types I and II and cytomegalovirus. Lactoferrin inhibits viral replication in the host cell rather than preventing virus entry for Hepatitis C Virus (HCV) and rotavirus. The high lactoferrin of camel milk is as a primary drug against HCV infection (Figure 1) [11].
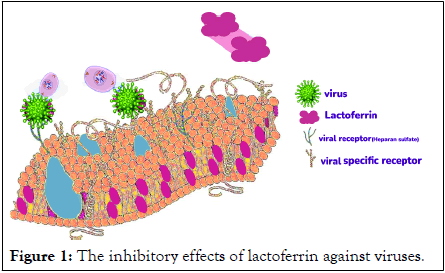
Figure 1: The inhibitory effects of lactoferrin against viruses.
One of the most hypotheses is that lactoferrin blocks viral receptor of heparan sulphate and inhibits infection.
The anti-viral effects of lactoferrin
When lactoferrin was added after viral penetration, virus adsorption or penetration by anti-HCMV activity of lactoferrin influenced. Antiviral activity of human and bovine lactoferrin was not different by pre-incubation of lactoferrin with HSV-1 or HSV-2 prior to infection, that declare antiviral activity of lactoferrin through interaction with cell surfaces is more than viral particles. Lactoferrin by binding to the virus particles prevents HSV entry.
Lactoferrin inhibition against viral infections is through interactions between viruses and glycosaminoglycan of host cells [12,13]. The strong negative charge of glycosaminoglycans lead to bind small cations, proteins, enzymes, growth factors, cytokines, chemokines, and lipoproteins, as well as some pathogens and viruses [14]. The viruses prevented by lactoferrin, don’t require heparan sulfate receptor on the cell for attachment. Lactoferrin reduces number of foci of Hantavirus infection of Vero E6 cells [15]. It appears that for some viruses such as HCV, the viral particle is main target for lactoferrin. It’s proved that camel lactoferrin completely inhibits HCV virus entry, but preincubation of lactoferrin with human leukocytes prior to HCV infection was not effective on viral entry [16]. Direct interaction between lactoferrin and the virus particle has been inhibited HIV-1 replication and syncytia formation depend to lactoferrin doses [17].
The lactoferrin inhibits replication of rota-, polio and adenovirus as naked viruses dependent to doses. Apolactoferrin bind to the rotavirus particle and prevent both hemaglutination and virus binding to cellular receptors and gradually inhibited by saturation with Fe3þ, Fe2þ. Mg2þ and Zn2þ, that the Zn2þ showed more inhibitory. Antiviral activity against poliovirus requires the presence of lactoferrin during the viral adsorption step, but zinc-saturated lactoferrin strongly inhibits viral infection after viral internalization.
It needs that lactoferrin to be added before or during the viral uptake for inhibition of adenovirus replication. Lactoferrin and adenovirus compete for viral glycosaminoglycan receptors on the target cells, which the N-labe is sufficient for inhibition [18].
Also, direct interaction between lactoferrin and viral proteins neutralizes adenovirus [19].
Human lactoferrin facilitates adenovirus entry into A549 cells rather than viral entry inhibition, that is not related to glycosaminoglycans on the cells or adenovirus receptor. It’s observed for bovine lactoferrin but much weaker than human lactoferrin.
A member of the Enterovirus family is Echovirus 6, can infect kidney cells of green monkey, which subsequently die due to apoptosis. Thus echovirus 6 inhibition is dependent on lactoferrin interaction with viral proteins rather than cellular glycosaminoglycans.
Lactoferrin modulates the host cell response to viral pathogens. Lactoferrin inhibits HIV attachment on dendritic cells and complexing with natural anti-HIV antibodies.
Also due to the induction of interferon-a/b expression by lactoferrin that result in inhibition of viral replication rather than prevention of virus entry or viral particles. Lactoferrin has synergy with antiviral drugs such as ribavirin to treat HCV [20], cidofovir against cytomegalovirus and zidovudine (an AZT analogue) against HIV.
Respiratory viruses: Bovine milk lactoferrin inhibited cell apoptosis by interfering in the caspase 3 function, also it blocked entry of ribonucleoproteins in H3N2 influenza A virus 5. Also, bovine lactoferrin in particular its C-lobe interacts with influenza A virus and prevent infection by different H1 and H3 viral subtypes viruses. According to the studies, lactoferrin inhibited RSV absorption and growth by blocking the internalization of viruses into the host cell but antiviral activity of lactoferrin will be decreased after processing.
Respiratory Syncytial Virus (RSV) is the most common cause of acute lower airway infections in infants and children. Breast milk components play a role in the antiviral effect against RSV. Lactoferrin control IL-8 secretion from cells that induced by RSV and hindered RSV uptake and infectivity.
Researchers revealed the lactoferrin directly interacted with F protein for penetration of RSV and occupied sites of protein for attachment of viruses. Lactoferrin has antiviral effects against human parainfluenza virus type 2 infection by preventing virus adsorption to the cells surface and limiting viral replication and infection.
It is confirmed that administration of lactoferrin by 100-1000 mg per day in humans reduced the incidences of colds and coldlike symptoms.
COVID-19: Control methods of COVID-19 pandemic are only containment and hygiene measures, while there are no antiviral treatments and vaccines yet. Its proposed nutritional supplements are useful against SARS-CoV-2 which causes COVID-19.
The spike protein of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) cause to virus entry into the host cells, so boosting the immune system will be useful against this virus. Milk of livestock in particular camel milk contains various protective proteins and enzymes such as lactoferrin which have immunological properties against the bacterial and viral infections. Milk lactoferrin has immuno-modulatory properties which strengthen host immune responses and prevent infections. Nutritional supplements are useful against COVID-19, but there are few clinical trials.
Lactoferrin as an anti-viral factor acts against viruses such as SARS-CoV(50); as 79% of sequences of the SARS-CoV and SARS-CoV-2 and also receptor-binding domain are homologous, therefore lactoferrin may inhibit SARS-CoV-2 invasion in a same manner to SARS-CoV.
The incidence of COVID-19 in infants was mild without ventilation support and lower respiratory tract infections rarely happened. Lactoferrin inhibited virus entry via binding to heparan sulphate glycosaminoglycan in the cell surface of human coronaviruses hCOV-NL63 and pseudotyped SARS-CoV CoV.Although if there are no published researches on lactoferrin effects on the SARS-CoV-2 entry into host cells but the interaction of lactoferrin with heparan cell receptors, which allow attachment on the cell surface in the primary phase of virus infections particularly in coronaviruses (Figure 2).
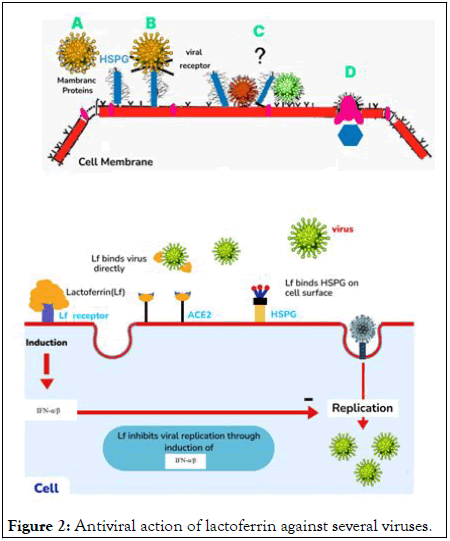
Figure 2: Antiviral action of lactoferrin against several viruses.
Lactoferrin prevents accumulation of viruses on the cell surface and inhibit the contact between the viruses and host cells and prevent the viral infection that is observed in the SARS-CoV epidemic and may be the same for SARS-CoV-2.
In some severe COVID-19 cases, mortality happens not only due to viral infection but also increases in cytokines and acute phase reactants such as interleukin IL-6, Tumor Necrosis Factor-α (TNF-α) and ferritin cause to mortality. In this case, lactoferrin reduce IL-6, TNF-α and downregulate ferritin.
Lactoferrin showed 50% inhibitory on human coronavirus of pseudo typed SARS-CoV that is most closely related with SARSCoV- 2 which causes COVID-19. Milk lactoferrin is effective for innate response to infections such as SARS-CoV-2.
Lactoferrin is widely used as an additive in infant formulas and in clinical studies applied various doses 100 mg to 4.5 g per day for various aims without apparent toxicities. Encapsulation and liposomalization as newer formulations of lactoferrin have been studied. Lactoferrin derivatives and peptides such as lactoferricin and lactoferrampin also have more potent antiviral properties.
One observation reported that relatively low incidence of COVID-19 infection in children may be relevant to lactoferrin. Incidence of COVID-19 in children 0-10 years old was only 0.9% in the Chinese cases. These cases were without requiring ICU or ventilation support. Also progressing of infection to lower respiratory tract rarely happened. Zinc saturation degree of lactoferrin can apparently exert a more potent antiviral effect particular relevance in COVID-19 as zinc supplementation has been proposed as a possible supplemental intervention for the disease.
Hepatitis C and B viruses: Hepatitis C Virus (HCV) is an enveloped virus belong to the flaiiridae family, that is single strand RNA and cause to persistent infection, is associated with chronic hepatitis, liver cirrhosis and hepatocellular carcinoma.
The antiviral effect of lactoferrin was against HCV is affected by heat treatment. hLF or bLf and peptides derived from lactoferrin probably interferes with adsorption of HCV to the host cells and binds to the E1 and E2 envelope proteins of HCV, and if pre incubated it will be most effective.
Complete inhibition of virus entry proved that when lactoferrin and HCV were pre-incubated. Pre-incubation with human leukocytes prior to HCV infection was not effective on viral entry.
bLF and hLf effectively prevented HCV infection in cultured human hepatocytes and bLf was most active. Lactoferrin also prevents HBV infection in a susceptible human hepatocyte cell line. Lactoferrin may be good anti-HBV candidates in patients with chronic hepatitis.
The effects of long-term oral administration of bLf in patients with chronic hepatitis C have showed that lactoferrin induced Th1-cytokine dominant environment in the peripheral blood and was effective on the eradication of HCV by interferon therapy. Lactoferrin by binding to lipopolysaccharides, preventing its attaching to CD14 (+) cells and resulted in reduced release of pro-inflammatory cytokines. Oral bLf about 1.8 g daily for 12 weeks, had significant effect in patients with chronic hepatitis C. Administration of bLf at higher doses (daily dose of 3.6 g instead of 1.8 g) for 8 weeks then bLf, interferon and ribavirin combined therapy for 24 weeks decrease HCV RNA titer by lactoferrin mono therapy but the effectiveness of the combined therapy of interferon and ribavirin in chronic hepatitis C patients observed.
It proved that purified camel lactoferrin interacts with HCV in Egyptian cases fed with camel milk which contains lactoferrin, that cause to complete virus entry inhibition.
Lactoferrin prevents HBV infection in cultured cells and pretreatment of cells with lactoferrin was required to inhibit HBV infection differently than HCV. As pre-incubation of HBV with bLf had not effect on viral infection. However, still it was not clear that bLf could inhibit HBV amplification in HBVinfected cells.
According to the researches, its reported that iron-, and zincsaturated bLf significantly inhibited the amplification of HBVDNA dependent to dose in HBV-infected HepG2 cells, while in this case, bLf hydrolysate were not effective.
Although breast milk provides bioactive components such as lactoferrin, it needs more studies that can partially explain the possible effect of breastfeeding on prevention of transmission of HBV from mother-to-child.
Human immunodeficiency virus: Human Immunodeficiency Virus (HIV) cause to AIDS, is belong to the lenti-iridae family. The genome including single stranded RNA that is packaged in a capsid which that is surrounded with an glycoproteins envelope that is important for the entry to the target cell.
Bovine and human lactoferrin are potent inhibitors of HIVinfection in vitro. Using of lactoferrin with zidovudine together had synergistic inhibitory effects on HIV. The antiviral mechanism of lactoferrin against HIV happens in early phase of infection, probably during virus adsorption to host cells and after infection the antiviral effects of lactoferrin reduced. Gp120 or chemokine receptors play an important role in the adsorption and entry of HIV into host cells by binding to CD4. Apo-LF was more effective than holo-LF against HIV. Succinylation of lactoferrin, enhanced antiviral effects against HIV-1 and HIV-2 but amination of lactoferrin cause to decrease of anti-HIV activity. Replication and syncytium formation of HIV-1 were efficiently inhibited depended to dosage by apo or holo Mn (II) and Zn (II)-lactoferrin, but not by ferric ion of lactoferrin. Studies proved that bLf strongly inhibited viral reverse transcriptase but only slightly prevented HIV-1 protease and integrase.
Bovine lactoferrin block HIV-1 infection using receptors of CXCR4and or CCR5, thus clearly inhibiting the HIV-1 entry to the host cells. Oral administration of bLf suppressed oral inflammation in cats infected with Feline Immunodeficiency Virus (FIV) with intractable stomatitis infection prior or during viral adsorption. Thus, bLf may have a potential application by modulating the cell proliferation, improve and protect functions of overactivated lymphocytes, cell cycle and cytokines expression as observed in cats in the end stage of FIV infection.
Herpes simplex virus: Herpes simplex virus type 1 and 2 (HSV-1 and HSV-2) are belong to of the herpes virus family. The genome of all herpes viruses including of DNA and infection with HSV can be persistent or latent. Reactivation of HSV-1 and 2 causes mild disease in immunocompetent cases. However, reactivations in AIDS-patients as immune compromised patients, transplant recipients and premature neonates can be quite severe and even life threatening. Antiviral effects of bovine and human apo-LF, holo-LF against both HSV-1 and 2 in the early phase of infections, HSV-1 in vitro, and in mouse cornea infection have proved. Even administration 1% LF solution significantly decreased infection, but virus replication was not completely inhibited. The saturation with Fe has no significant role in the inhibition of HSV.
Lactoferrin also binds heparan sulfate as one of the most important glycosaminoglycan for virus with high affinity as it has two glycosaminoglycan-binding domains in N-lobe, and likely its responsible for preventing viral HSV-1 entry.
Heparan sulfate is important for lactoferrin antiviral activity against HSV. According to studies, there was no difference in the inhibitory ability of lactoferrin for viral entry when preincubated with the cells prior to infection or after viral attachment (1 h at 4°C).
The initial attachment of HSV to cells occurs by binding to the viral glycoproteins (gC or gB) or heparan sulfate on the cell surfaces. In the absence of heparan sulfate, virus binds to chondroitin sulfate proteoglycans on the target cells but with lower efficiency. The anti-HSV-1 activity of lactoferrin is not related to attachment to cell glycosaminoglycan such as heparan sulfate and chondroitin sulfate.
The inhibitory effect of bovine lactoferrin against HSV-2 entry is not due to interference with attachment of viral glycoprotein C with heparan sulfate that is different with HSV-1. Heparan sulfate and other glycosaminoglycans play important roles in viral entry, the rapid partial internalization, and HSV cell-to-cell spread that are key mechanisms for viral escape from the host immune response.
Both human and bovine Lf (independent to iron or sialic acid content) inhibits infection and replication of HSV-1 in lung cells of human embryo. bLf, Mn-bLf and Zn-bLf are potent inhibitors against HSV-1 and HSV-2 infections by binding to host cells and HSV particles. It proved that the part of N-lobe (residues 1–280) and one peptide of C-lobe were ten-times more effective than another peptide in the N-lobe and this part of Clobe was six-time less active than native bLf.
Lactoferrin inhibited cell-to cell spread of HSV-1. Human lactoferrin inhibits cell-to cell spread with less effectively than bovine lactoferrin and for HSV-2 was less. bLf was more effective than the hLf against HSV-2 infection, thus lactoferrin an adjunct treatment against herpes virus activity and herpetic infections. The tryptic digestion of lactoferrin led to different fractions from the C and N-lobe with antiviral activity. Inhibitory effect of Lfcin was dependent on the presence of heparin sulfate at the cell surface that proves its antiviral activity, thereby blocking viral entry and cell-to-cell spread of both HSV-1 and HSV-2. However, inhibition of cell to-cell spread by bovine Lfcin depend to chondroitin sulfate of cell surfaces.
Cytomegalovirus: Cytomegalovirus is belonged to the-herpes virus family. It causes a latent and persistent infection. But CMV often happens during the early years of life and generally due to the lack of clinical symptoms, primary infection is not clear. CMV can reactivate in the immune deficiency cases such as AIDS patients, transplant recipients or pre-term neonates and led to severe morbidity and mortality. Pre-incubation of target cells with lactoferrin probably prevent the virus entry and attachment to the target cells by low affinity binding to heparan sulphate proteoglycans. Deletion of the Arg in N-lobe (responsible for binding to HSPGs) diminishes the antiviral activity of lactoferrin. The affinity of lactoferrin was increased by amination, but succinylation reduced the antiviral effect of lactoferrin.
Lactoferrin protected Murine CMV (MCMV) against lethal infection. The antiviral effect of lactoferrin was proper when it was added prior to infection. Also, protective effects of lactoferrin were due to an upregulation of Natural Killer cells (NKcells), monocytes and granulocytes which eliminated the infection. Protective effect of lactoferrin in the transmission of HCMV to the newborn happens during the first month postpartum.
Both lactoferrin and lactoferricin prevented HCMV entry into the host cells in the early steps of cytomegalovirus infection.
Antiviral activity of hLF and bLf against human CMV is independent to lactoferrin iron or sialic acid. Lactoferrin cause to 10-fold reduction in the final virus titres at 4 weeks after infection in immunocompromised rats and lactoferrin effects take place by inhibition of viral entry more than boosting of the immune responses.
Rotavirus: Rotavirus is belonged to the Reoiridae-family and genome consists double stranded RNA, enveloped within a three-shelled capsid. Rotavirus infections are most important cause for non-bacterial gastroenteritis in neonates and children are approximately 1 million death every year.
Main reason of severe diarrhea in children under five years old is rotavirus infections [4]. Apo and holo bLf inhibit the replication of rotavirus and simian Rotavirus SA11 in vitro independent dose and apo-Lf was the most active.
Manganese or zinc-saturated bLf decreased activity compared with apo or holo bLf, and the deletion of sialic acid increased the anti-rotavirus activity. Tryptic fragments of bLf; 86–258 and (324–329: YLTTLK) could inhibit rotavirus lower than native bLf.
The antiviral mechanism against Rotavirus is due to prevention of adsorption of the virus to the target cells by binding to virus particles, host cells and attachment of virus to viral receptors on the host cells is inhibited. Rotavirus cannot bind glycosaminoglycans (heparan sulphates), thus lactoferrin cannot compete with rotavirus for binding to cellular receptors.
Lactoferrin prevented antigen synthesis of Rotavirus during infection in intestinal cultured cells. Thus, prevents infection, maintains antiviral effects after the virus entry into host cell. But some researchers reported lactoferrin was not effective on Rota virus.
Since the rotavirus strain binds to glycidic residues and viral particles not glycosaminoglycans, but prevention of viral attachment by blf was not related to a competition for binding sites on HT-29 cells. The bLf inhibition after adsorption related to the calcium, as key agent for the virus morphogenesis. Other studies investigated the role of metal binding, sialic acid and tryptic fragments of bLf against Rotavirus infection.
The effect of whey protein with or without lactoferrin on rotavirus infection in suckling rats reduces the severity of acute gastroenteritis of rotavirus and stimulate the immune responses.
Adenovirus: Adenovirus infection prevented by both bLf and hLf dependent to dose in vitro, but metal-saturated bLf did not significantly affect its activity against adenovirus infection. bLf prevented the early step of viral infection, hindering adenovirus antigen synthesis when pre incubated or when added during the attachment. bLf and Lfcin activity was stronger than hlf and only the N-lobe had effect and C-lobe was without effect.
This antiviral activity of bLf happens through interaction with the protein responsible for viral attachment to the integrin cell receptors or adenovirus penton base. The primary receptor for infection of most adenovirus species (A, C, D, E and F) was the coxsakievirus adenovirus receptor. Moreover, adenoviral infection was enhanced by bLf but not hLf.
Due to the high concentration of free lactoferrin in tears about 2.2 mg/mL, likely that lactoferrin has protective role against viral adhesion and pathogenesis. Commercial hLf (Sigma- Aldrich), or bLf or recombinant has promoted adenoviral infection of corneal epithelial cells. Indeed, hLf and bLf, in native and apo form, and Fe3+-, Mn2+- and Zn2+- saturated bLf, added before or during the viral adsorption, or the entire replicative cycle, were effective against adenovirus type 2 infection in cultured cells. The N-lobe unlike the C-lobe can bind to heparan sulfate in competition with viral receptors of adenovirus type 2.
Hantavirus: Hantavirus focus formation inhibited by preincubation of cells with bLf before infection by 85%, but post infection the focus formation inhibited by 97.5%. bLf pre and Rbv post-treatment were evaluated in suckling mice infected with hantavirus, that both lactoferrin and Rbv were efficacious against hantavirus infection in vivo.
Polio virus: Poliovirus is an enterovirus belong to the Picorna_Iridae family. These viruses have small genome, consisting single stranded positive RNA, which is packaged in a small single capsid without any envelope. Infections with poliovirus lead to poliomyelitis, which can cause paralysis of limbs. Lactoferrin during infection with poliovirus, had antiviral function against poliovirus in the early phases of viral infection and prevent virus entry of into the target cell and inhibit viral replication.
Echo virus: hLf and bLf inhibit the cytopathic effect of Enterovirus 71 (EV71) on human embryonic rhabdomyosarcoma cells. Lactoferrin effect probably through inhibiting of viral adsorption. Echovirus 6 infected cells die as a result of apoptosis that bLf inhibited programmed cell death.
Anti-echoviral mechanism is due to prevention of viral genome entry into the cytoplasm likely lactoferrin interact with echovirus capsid proteins, cell surface glycosaminoglycan chains and induces alterations that stabilize the conformation of the virion and resistance to uncoating. The increasing of net negative charges of lactoferrin by acylation destroyed antiviral effects.
Friend Virus Complex (FVC): A murine retrovirus, causes an erythroleukemia in mice within 3 months after infection proved the antiviral effects of human lactoferrin against FVC in mouse leukemia model and Human lactoferrin decreased virus titres in the infected mice spleen.
Administration of lactoferrin as intraperitoneally in the early phase of infection and even a single bolus injection within 2 h after infection was effective. Since lactoferrin had no direct effect on FVC infection in vitro, thus the antiviral activity in these animals probably was due to the regulatory effect of lactoferrin on the myelopoiesis that decrease myelopoiesis in bone marrow and the spleen and able to act as transcription factor. It is proved that lactoferrin decrease cycling status of hemopoietic progenitor cells. Results of in vivo study proved that the protective effect of holo lactoferrin was probably due to action on cells responding to the FVC or on cells which influence the virus.
Human Papillomavirus (HPV): Lactoferrin was effective in the HPV uptake process dependent to dose and bLf was more potent inhibitor of HPV entry than hLf. bLf and hLf were potent inhibitors of HPV-5 and 16 infections (ex115ora). Moreover, bovine and human Lfcin had antiviral effects and efficacy was depending on size, charge and structures of the Lfcin.
Livestock milk lactoferrin can modulate immune responses to viral infections by binding to virus particles or receptors and may it act against viral attacks and reduce the severe infections, so it could be an adjunct treatment for more severe cases of COVID-19. Immuno-modulatory, anti-bacterial and antiviral properties of lactoferrin make it unique preventative agent against viral infections. But it needs more studies to verify dosage and efficacy of lactoferrin on prevention and treatment.
Therefore, lactoferrin as powder or tablets can be a novel promising method against viral infections and application as a drug carrier. According to this point that camel milk has highest amount of lactoferrin in comparison with the other milks, so antiviral activity will be high as well, therefore it will be a precocious source of lactoferrin against the viruses.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Mohammadabadi T, Bouyahya A (2025) Milk Lactoferrin: A Nutraceutical Supplement against Viruses. J Nutr Food Sci. 15:077.
Received: 10-Oct-2024, Manuscript No. JNFS-23-27298; Editor assigned: 12-Oct-2024, Pre QC No. JNFS-23-27298 (PQ); Reviewed: 26-Oct-2024, QC No. JNFS-23-27298; Revised: 23-Feb-2025, Manuscript No. JNFS-23-27298 (R); Published: 01-Mar-2025 , DOI: 10.35248/2155-9600.25.15.077
Copyright: © 2025 Mohammadabadi T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.