Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2019)Volume 9, Issue 3
There is substantial data that fermented products have a diverse range of health benefits ranging from immunomodulation, improving lipid profiles and more recently positive effects on the microbiome. Despite these desirable clinical attributes there are practical barriers to the use of fermented products. Two such barriers are that many studies were performed using experimental products, and lacked careful characterization of content, stability, toxicology and safety. To overcome these issues, we identified a widely available fermented product (BESO Q-CAN® Plus) and i) conducted independent analysis of total isoflavone content, aerobic bacteria, yeast and mold plate counts, quantification of heavy metals, solvents, and ii) performed a safety clinical trial in 10 lean and 10 obese healthy subjects who consumed Q-CAN® Plus for 4 weeks, with a further 8 week follow-up.
Q-CAN® Plus had 120.9 mg/100 ml of total isoflavones. Bacteria, mold, yeast were all below the normal limits. A profile of 300 herbicides, pesticides, fungicides and solvents was negative. Lead, mercury, arsenic and cadmium were below the detectable limit. A 24-month stability study demonstrated no decrease in the concentration of isoflavones and no microbiological contamination. The clinical trial demonstrated that Q-CAN® Plus was well tolerated.
This independent analysis of Q-CAN® Plus demonstrated a stable isoflavone content, absence of a wide range of potential toxins and stability over 24 months. Q-CAN® Plus was also well tolerated. These data provide the information required for the safe use of an easily available fermented soy product.
Fermented; Soy; Stability; Toxicology; Safety
AOAC: Association of Analytical Communities
Fermented foods have been part of the diet for thousands of years, and fermentation was initially performed to allow preservation of food, and perhaps to enhance flavor [1]. Fermentation has generally been defined as food items made from controlled microbial growth and enzymatic conversions of major and minor food components [2]. In the last 50 years a large amount of data has accumulated demonstrating that fermented foods, in addition to having a longer shelf life, also have a wide range of health benefits [3,4]. Food fermentation has a limited number of components including the starting food substance, the microorganism(s) involved and the major metabolite produced by them (such as lactate, acetic acid, ammonia). A wide range of foods have been fermented, and approximately a dozen major organisms are commonly used as fermenters. This along with different fermentation conditions has resulted in a vast range of fermented food products. This high level of variety, and typically little characterization, has made it very challenging for individuals wanting to avail themselves of the benefits of fermented foods to do so in a reliable and safe manner [4-6].
Our goal was to undertake a detailed characterization of a widely available fermented food for the purposes of determining if it was safe from the presence of microorganisms, naturally found heavy metals and artificial compounds such as solvents, pesticides and fungicides. One study evaluated the third-party quality control testing in 1.5 million dietary supplement prescriptions representing over 753 unique products in the department of defense military treatment facilities. It was found that <3.6% of all products examined were third-party certified/verified by recognized national organizations [7]. In addition, we wished to test if it was stable over a 2-year period at room temperature and high temperature conditions. This is the type of data that is needed before the beneficial effects of fermented food products can be distributed beyond a few enthusiasts and early adopters. For this purpose, we selected the fermented product Q-CAN® Plus. This was selected because it has been widely used in China, and is also available in the United States. In addition, it uses the well-known starting food item soy, which also allowed us to test for the presence of isoflavones which are known to have a wide range of health benefits.
Finally, we conducted a clinical trial with Q-CAN® Plus to test if there were either any undesirable clinical or hematological consequences of consuming Q-CAN® Plus over a four-week period, with a further eight weeks of follow-up. As this was the first formal safety characterization we enrolled healthy individuals, and due to the high prevalence of obesity in the population, we enrolled both lean and obese groups. The combination of extensive characterization, and a clinical safety study provides the information needed by individuals who may wish to investigate potential beneficial effects of Q-CAN® Plus.
Q-CAN® plus analysis
Shelf and controlled chamber testing: Testing for isoflavone content and microbiological analysis was performed at baseline, 12 and 24 months at room temperature and also at high temperature (40°C).
Total soy isoflavone content was measured utilizing the Isoflavone (ASOF_S:11) assay. Official Methods of Analysis of AOAC INTERNATIONAL, (2005) 18th ED., AOAC. INTERNATIONAL Gaithersburg, MD, USA, Official Methods 2001.10.
Soy GMO Status was determined via Qualitative PCR assays performed for CaMV 35S promoter and MON89788 RR2 Soybean.
Microbiology Profile was determined via Microbial Limit Tests USP 31. Evaluation of Aerobic Plate Count (61), Enterobacteriaceae (62), E. coli (62), Salmonella (sp) (62), Culture for Staph aureus (62), Culture for Pseudomonas aeruginosa (62), Total Mold and Yeast Count (61). Aflatoxins B1, B2, G1 and G2 were evaluated via HPLC AOAC OMA 991.31.H.
Heavy metal elements: Lead, Mercury, Arsenic, Cadmium were evaluated via ICP Mass Spectrometry (ICP_MS_S:21) Official Methods of Analysis, Method 2011.19 and 993.14, AOAC INTERNATIONAL. Assay detectable limit of 5 to 10ppb.
Total Pyrethrum and pesticides assayed via Multi-Residue Screen for spices, botanicals, harvested products (PS03_SPL:11). AOAC Official Method 2007.01 and CEN Standard Method EN 15662.
Residual solvents: A panel of 52 chemical were assayed utilizing Residual Solvents (RESO_S:12) United States Pharmacopeia, 32nd Rev. - National Formulary 27th Ed., Method <467>, USP Convention, Inc., Rockville, MD (2009). Solvent Residues were compared to the standard LOQ (Limit of Quantification) Bertsch, Brian. "Developing One Universal Method for Residual Solvents Using the New Teledyne Tekmar HT # Headspace Sample Introduction System", Application note (Teledyne Tekmar), Document # HT #-001. September 2005.
Ethics and trial registration
Once the manufacturer’s Certificate of Analysis was verified through independent laboratory analysis, the study protocol was submitted and approved by the Yale University Institutional Review Board/Human Investigation Committee (New Haven, CT). All subject data were collected at the Yale Center for Clinical Investigation’s Outpatient Research Facility (New Haven, CT). All subjects provided written informed consent and received financial compensation. The study protocol was registered on ClinicalTrials.gov prior to enrollment (NCT02656056).
Clinical trial
A prospective 14-week clinical trial was conducted in 20 healthy adults, with no known allergy or sensitivity to soy or soy products. A three-week pre-soy lead-in phase was followed by four weeks of consuming two 236 ml bottles of Q-CAN® Plus per day (472 ml total, ~ 16 oz.), followed by an eight-week post-soy follow-up period as shown in Figure 1. There were a total of 10 lean and 10 obese subjects, with 11 clinic visits over 14 weeks.
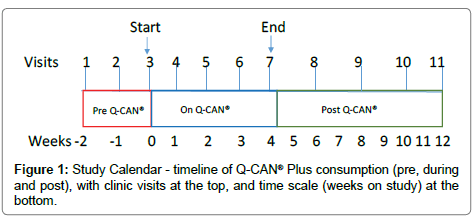
Figure 1: Study Calendar - timeline of Q-CAN® Plus consumption (pre, during and post), with clinic visits at the top, and time scale (weeks on study) at the bottom.
Inclusion criteria: Adults 18-70 years of age with no major health complications: 10 within the lean BMI range (18.5-25), and 10 within the obese BMI range (30-40).
Exclusion criteria: Individuals allergic to milk protein and soy or soy derivatives were excluded. Also excluded were individuals with a history of inflammatory bowel disease, gastrointestinal bleeding, radiation proctitis, or abdominal surgeries (other than cholecystectomy, appendectomy, hysterectomy, and hernia repair). Individuals were also excluded if they recently (within 30 days) used antibiotics, probiotics, anticholinergics, opioids or other analgesics (with the exception of nonsteroidal anti-inflammatory drugs), anti-coagulants, or immunosuppressives. Individuals with alcohol use disorder (or consumed three drinks per day) or who reported major changes in dietary habits in past six months were excluded from participation. Anorexia nervosa, autoimmune disease, bulimia, celiac disease, chronic infections, organic causes, and illicit drug use also were conditions for exclusion. We excluded women who were either pregnant or intended to become pregnant during the course of the study, as pregnancy often is associated with increased sensitivity to odors or food tastes, which could impact adherence in consuming fermented soy products which are known for their distinctively strong taste and odor. Use of tobacco was excluded (e.g., cigarettes, smokeless tobacco, cigars and pipes) within 30 days of enrollment.
Information collected: During each study visit, subjects’ current weight was recorded. During the intervention period, subjects were asked whether they noticed any changes since the previous visit to assess side-effects of consuming fermented soy.
Biosafety assays: Blood was collected at weeks -2, 0, 2, 4, 6, 10, 12, and the following hematological parameters quantified: mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean platelet volume (MPV), red blood cell distribution width (RDW-CV), hematocrit, RBC count, WBC count, % neutrophils, average number of neutrophils (# neutrophils), % monocytes, % basophils, average number of lymphocytes (# lymphocytes), % eosinophils and C- reactive protein were analyzed.
Data analysis was conducted for the three phases of the clinical trial. Statistical analysis was performed employing paired t-test and one-way ANOVA (significance of p ≤ 0.05).
Q-CAN® plus analysis
Quantification of isoflavone content revealed that at baseline the total isoflavone content was 120.9 mg/100ml. For all four subsequent testing conditions (12 months room/high temperature and 24 months room/high temperature) there was no significant change in the concentration of total isoflavones.
Aerobic microbiological profile examined the total aerobic microbiological count, and also individual counts for Enterobacteriaceae, E. coli, Salmonella (sp), Staph aureus, pseudomonas aeruginosa. In addition, the counts for molds and yeast were also quantified (Table 1). At baseline all counts were either extremely low (below 10 colony forming units per gm) or were undetectable, and these did not change at 12 and 24 months at the two testing temperatures.
| Analyte microbiology profile | Baseline | Pass/Fail | 12Mo-C † | 12Mo-S ‡ | 24Mo-C | 24Mo-S |
|---|---|---|---|---|---|---|
| Total aerobic microbial count | <10 CFU* /gm ! | Pass | Pass | Pass | Pass | Pass |
| Enterobacteriaceae | <10 CFU /gm | Pass | Pass | Pass | Pass | Pass |
| E. coli | Non-detected | Pass | Pass | Pass | Pass | Pass |
| Salmonella (sp) | Non-detected | Pass | Pass | Pass | Pass | Pass |
| Staph aureus | Non-detected | Pass | Pass | Pass | Pass | Pass |
| Pseudomonas aeruginosa | Non-detected | Pass | Pass | Pass | Pass | Pass |
| Mold and Yeast | <10 CFU /gm | Pass | Pass | Pass | Pass | Pass |
Table 1: Quantification of bacteria, yeast and molds.
The environment contains several heavy metals that can be toxic and in addition food processing can introduce solvents which may have associated toxicities. Analysis of the heavy metal content for arsenic, cadmium, lead and mercury and 25 solvents at the baseline time point revealed that all had concentrations that were well below the safety levels established by The Association of Analytical Communities (AOAC) (Table 2).
| Analyte | Limit | Result | Pass/Fail |
|---|---|---|---|
| Arsenic | (5 to l0ppb) | <0.0100 ppm | Pass |
| Cadmium | (5 to l0ppb) | <0.0100 ppm | Pass |
| Lead | (5 to l0ppb) | <0.00500 ppm | Pass |
| Mercury | (5 to l0ppb) | <0.0100 ppm | Pass |
Table 2: Quantification of heavy metals.
Clinical trial
Demographic characteristics of the trial subjects (n = 20) are shown in Table 3.
| Demographic Characteristics | Lean (n=10) | Obese (n=10) |
|---|---|---|
| Gender (n) | ||
| Female | 7 | 3 |
| Male | 3 | 7 |
| Age (mean, SD) | 32.2 ± 12.23 | 44.5 ± 13.75 |
| Weight (mean, SD) | 136.25 ± 22.42 | 221.90 ± 29.13 |
| BMI (mean, SD) | 21.85 ± 2.01 | 33.90 ± 1.75 |
| Race (n) | ||
| White | 8 | 5 |
| Black | 1 | 2 |
| Unknown | 1 | 3 |
| Ethnicity (n) | ||
| Non-Hispanic | 6 | 7 |
| Hispanic or Latino | 4 | 2 |
| Unknown | 0 | 1 |
| Co-morbidities/diagnoses | None | Depression (1) Type 2 DM (1) Hypertension (2) Hyperlipidemia (1) |
Table 3: Demographics table – includes gender, age, weight mean BMI, race, ethnicity and diagnoses reported in subjects in the obese arm.
There were no side-effects or other reportable events other than some reports of mild gastrointestinal symptoms.
The hematology results show no significant change in any of the tested parameters (mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), mean platelet volume (MPV), red blood cell distribution width (RDW-CV), hematocrit, RBC count, WBC count, % neutrophils, average number of neutrophils (# neutrophils), % monocytes, % basophils, average number of lymphocytes (# lymphocytes), % eosinophils and C- reactive protein were analyzed). The values for hemoglobin and mean platelet volume are shown in Figure 2 as an example.
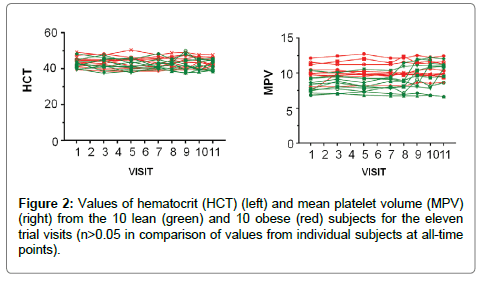
Figure 2: Values of hematocrit (HCT) (left) and mean platelet volume (MPV) (right) from the 10 lean (green) and 10 obese (red) subjects for the eleven trial visits (n>0.05 in comparison of values from individual subjects at all-time points).
Fermented foods have been routinely consumed in most regions in the east for hundreds of years, and in the last few decades there has been a rapid expansion in the experimental and clinical data supporting the health benefits of fermented food items beyond simple nutrition. Such health benefits include lowering serum cholesterol, improving the intestinal microbiome and potential anti-cancer effects [3,4]. Despite this growing evidence of the health benefits of fermented foods there has been limited clinical trial work on fermented foods, and they have not become a routine food item in Western industrialized countries. This particularly surprising because the cardiometabolic and anti-cancer effects of fermented foods are particularly relevant in industrialized countries. Part of the reason for this may be that the category of fermented foods includes a highly heterogeneous set of food products, and these have not been carefully characterized for their composition, chemical and clinical safety. Furthermore, many published studies were conducted on experimental fermented foods which cannot be reliably duplicated.
To facilitate clinical studies in the area of fermented foods, and to provide chemical and clinical safety data we performed an extensive set of laboratory and clinical analysis on the fermented food product Q-CAN® Plus. We chose this product because it already has a track record of use in China and is easily available in the US for clinical and other studies.
As culture of microorganisms in the food item is an essential step in the fermentation process, we started by quantifying the microbial count (bacteria, yeast and molds). Q-CAN® Plus, like many fermented foods, undergoes sterilization after fermentation. As expected a wide range of bacteria, yeast and molds were either absent or had colony forming units that were at the lower limit of detection (CFU<10/gm), and these did not increase even after storage for 24 months at room temperature and 40°C. By comparison the CFU in human saliva is approximately 107/gm [8]. This is important information for everyone considering a clinical trial, and for individuals who would like to include it in their diet. It has further relevance as fermented products such as Q-CAN® Plus are often consumed by patients with chronic illnesses, or who are recovering from an acute illness, when the intestinal barrier or the immune system may be compromised, and it would not be desirable to expose the patient to any products with a high bacterial count.
In addition to microorganisms, heavy metals such as arsenic, cadmium, lead and mercury are a potential health risk [9,10]. These metals are found in nature and can also be present in containers and other items that come into contact with fermented foods. Arsenic toxicity is most commonly associated with contaminated wine, and in some herbal products arsenic has been intentionally added in the hopes of a therapeutic response [11,12]. Cadmium is typically present in non-food items but can still be ingested when soil or foods are contaminated by chemical stabilizers and fertilizers [13]. Lead and mercury exposure is usually an industrial hazard but can enter the food and water cycle [14]. The quantification of each of these demonstrated levels that were below 0.01 ppm, which is 500 times below the lower limit of the acceptable level. The very reassuringly low levels of these heavy metals are important in establishing the safety of Q-CAN® Plus. A wide range of solvents are used in industrial processes and can enter the food chain. These can cause a range of toxicities including neurological, skin and liver. As for heavy metals the concentration of solvents was very low for the 25 solvents tested.
Q-CAN was well tolerated, and these clinical findings were supported by hematological analysis which over the 14-week course of the study which did not find any significant changes in the parameters analyzed (data for hematocrit and mean platelet volume shown in Figure 2).
In this study we have extensively evaluated the content, safety and stability profile of Q-CAN® Plus. The results establish that it is a product with an excellent and safety profile, and furthermore has also found to have clinical and laboratory safety in a trial of 10 lean and 10 obese subjects. These data allow for the consideration of Q-CAN® Plus in clinical trials of more disease-targeted indications.
This project was funded by BESO Biologic Research.
Citation: Mankash SM, Arumugam S, Dioletis E, Paiva R, Secor ER, Weiss TR, et al. (2019) Isoflavone Stability, Quality Control Assessment and Clinical Safety of Q-CAN® Plus, a Novel Fermented Beverage. J Nutr Food Sci 9:757.
Received: 18-Mar-2019 Accepted: 15-Apr-2019 Published: 24-Apr-2019 , DOI: 10.35248/2155-9600.19.9.1000757
Copyright: © 2019 Mankash SM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : This publication was made possible by BESO Biological Research Inc. and funding from the NIH, CTSA Grant Number UL1 TR001863 from the National Center for Advancing Translational Science (NCATS). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.