Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2012) Volume 2, Issue 3
Although oxygen is essential for aerobic life forms, the high prooxidant activity of its metabolites can cause cell injury. Mitochondria which consume more than 90% of the oxygen received by an organism are the primary source of free radicals [1]. Other sources of free radicals include pro-oxidative enzymes liberated during stress, inflammation or immune activity, [1] and environmental factors such as air pollution, sun radiation and chemicals [2]. Although most oxidant/antioxidant reactions occur within organ cells, pro-oxidants and antioxidants along with all byproducts are transported through the bloodstream in which erythrocytes are directly exposed to free radicals. Endogenous antioxidant defense systems protect the body by continually balancing free radicals in cells and in the blood [2,3]. However, the efficiency of such mechanisms tends to deteriorate for diverse reasons including aging, disease or poor nutritional habits. In these specific situations, dietary antioxidant compounds could play a significant role in keeping free radicals at or under a normal physiological level [2-6].
Consequently, quantifying a food’s antioxidant potential has become an important issue in food science research. The various methods to analyze antioxidant potential can be separated into at least three different categories: In Vitro methods that involve exclusively chemical reactions such as DPPH (2,2’-diphenyl-1-picrylhydrazyl) free radical scavenging potential, Ferric Reducing Antioxidant Power (FRAP), Trolox Equivalent Antioxidant Capacity (TEAC), Total Reactive Antioxidant Potential (TRAP), and Hydrophilic Oxygen Radical Absorbance Capacity (H-ORAC) among others, In Vitro methods that involve living cells, and in vivo methods which measure the antioxidant capacity of the food metabolome obtained from living organisms after food ingestion. From the health perspective, in vivo methods are obviously the most relevant, but they require too many resources to be used as screening methods. Some dietary antioxidant compounds are often converted before absorption and consequently undergo important transformations that modulate their in vivo biological activity. Nonetheless, screening of food extracts using simple methods is necessary and then more profound studies should focus primarily on the food products with the highest potential to exert in vivo biological activities. Furthermore, because food has direct contact with epithelial cells before absorption, tests on food extract could be also useful to screen foods that potentially protect the gastrointestinal tract.
Because they are rapid and cost-effective, chemical assays provide the largest source of information about the antioxidant potential of food. Indeed, the semi-automated H-ORAC assay has become a standard method for measuring antioxidant potential and extensive databases have been built mainly for Western food products [7-9]. However, to discover whether different screening assays may provide superior information about the antioxidant potential of food, it will be necessary to complement such databases with antioxidant assays that do not rely on chemical reactions alone. An additional step towards more biologically relevant screening assays is the use of living cells to model potential cell-related antioxidant activities. Methods that involve living cells however are often intricate, expensive, and difficult to automate mostly because of the complex and painstaking process of cell culture.
Because erythrocytes are robust and easily available and do not require culture, they have been extensively used as a model to study cell-related effects of antioxidant compounds [2,10-19]. In addition, the use of erythrocytes is a biologically relevant model to study the antioxidant potential of phytochemicals because they are major targets of free radical attack in the blood. When the bloodstream removes oxygen from the lungs, free oxygen radicals species result from respiration metabolism. Indeed, red blood cells are primarily involved in oxygen transportation, and as a consequence, they can also scavenge reactive oxygen and nitrogen species.
Because all previous methods that have used erythrocytes include multiple steps, they cannot be easily automated to analyze a larger number of biological extracts. However, the Erythrocyte Cellular Antioxidant Assay (ERYCA) has recently been developed for such a purpose [20]. Like the H-ORAC method, this method can be semiautomated in a cost effective manner. It is based on the inhibition of lipid peroxidation of erythrocyte membranes and subsequent hemolysis, which is induced by free peroxyl radicals released by AAPH in the same way that H-ORAC is based on the inhibition of fluorescein oxidation. Unlike other methods using erythrocytes, ERYCA continuously assesses the kinetics of hemolysis by registering the loss of light scattering properties of lysed cells. The method was found to be relatively fast, sensitive, accurate and repeatable even when using erythrocytes from different donors and various storage times up to 48 days after blood collection [20].
In contrast to H-ORAC, the ERYCA assay has the advantage of assessing different mechanisms of antioxidant protection, including direct scavenging of free radicals in the surrounding medium and cellmediated antioxidant protection (Cell-MAP), in one step. Cell-MAP addresses the following: the physicochemical properties of antioxidants such as their liposolubility [14], the ability of both water and lipid soluble compounds to diffuse effectively into lipoproteins and cell membranes [2,15] and eventually enhance from there, the erythrocytes defenses through mediation of both, plasma membrane redox system (PMRS), and the antioxidative defense enzyme system [10-12,21-23].
Even though the mechanism by which exogenous antioxidant compounds can enhance the antioxidant protection of cells is not fully understood, it has been shown that phenolics such as quercetin can penetrate into the membrane phospholipidic bilayers [15]. From there phenolics act as an intracellular substrate of PMRS oxidoreductase enzymes [10,21,24] and can donate electrons to extracellular electron acceptors through the erythrocyte PMRS [10]. Phenolic compounds can be absorbed in erythrocyte membranes in much higher concentrations than in the surrounding media [12,15,23]. In this way, they can help “fuel” the transmembrane electron transfer system, which would otherwise be limited by the initial capacity of the endogenous intracellular electron donors. It has been suggested that this could be the main mechanism by which dietary flavonoids enhance cell protection against antioxidative stress and exert their potential health effects [10].
In the ERYCA assay, it cannot be determined which part of the antioxidant protection observed is due to cell-related processes or to direct quenching of free radicals in the surrounding media. Nonetheless, by comparison with the H-ORAC assay, which exclusively analyzes peroxyl-radical-scavenging activity, the relative importance of cell-related process in antioxidant protection can eventually be demonstrated. Consequently, the ERYCA assay, in combination with H-ORAC can improve screening strategies for assessing the antioxidant potential of food.
This study quantifies the global antioxidant protective effect on erythrocytes of 34 common tropical fruit extracts and compares the results with H-ORAC to study cell-mediated antioxidant protection.
Reagents
The following reagents were obtained from Sigma-Aldrich Co. (St. Louis, MO): quercetin (C15 H10 O7 ), Trolox (6-hydroxy-2,5,7,815 10 7 tetramethylchroman-2-carboxylic acid), gallic acid (C7H6O5), fluorescein sodium salt (C20 H10 Na2 O5 ), Folin–Ciocalteu reagent, sodium carbonate (Na2CO3), and reagents for making phosphatebuffered saline solution (PBS). Also 2,2’-azobis (2-amidinopropane) dihydrochloride (AAPH) from Wako Chemicals USA, Inc. (Richmond, VA) and acetone from Mallinckrodt Baker, Inc. (Phillipsburg, NJ) were purchased. Blood samples (O Rh negative) from healthy human donors were provided by a Costa Rican blood bank after the samples’ shelf life had expired (on average, 30 days after blood collection).
Preparing fruit samples
The most frequently fruits consumed in Central America and the Caribbean were selected. The fruits were sampled principally from retail outlets in local markets and also directly from farms. Approximately 1 kg of each fruit was randomly selected. Upon arrival at the laboratory, the fruits were immediately processed to remove non-edible parts, such as the peel and seeds. Green coconut water was extracted directly from the fruit cavity. Oranges and mandarin limes (a hybrid also known as Rangpur lime) were hand-squeezed and filtered through a 1-mm sieve. Coffee cherry was pressed to obtain juice. Plantain, chayote, and peach palm fruit were prepared and boiled in water for 12, 10, and 60 min respectively. Ripe plantain was deep-fried for 5 min in oil at 160°C. Rice and beans were also cooked as usual.
All recovered edible parts were immediately frozen in liquid nitrogen, freeze-dried, powdered in a hammer mill, vacuum-packed into laminated bags, and stored at 40°C before analysis. For analysis, 0.5 g of freeze-dried powder was extracted using 20 ml aqueous acetone solution (30/70 v/v) for 15 min and magnetic agitation at 60-80 rpm, followed by 15 min in an ultrasound bath and then another 15 min of magnetic agitation. Each step was performed at room temperature. The slurry was filtered through Whatman Filter Paper No. 41, the residue was re-suspended once again in 20 ml aqueous acetone, and the extraction procedure was repeated once more. The fat in the peach palm and avocado was extracted with ether before analysis.
All recovered filtrates were concentrated at 40°C in a rotary vacuum evaporator to remove potentially toxic acetone, and after 0.45 mm filtration, the extract is resuspended in water to a 25 ml final volume. This solution was labeled “the fruit extract”. All processes for obtaining fruit extracts were performed independently in duplicate.
Erythrocyte cellular antioxidant assay
The ERYCA assay protocol was performed as previously described [20], with the exception of a modification for the present study by introduction of 15 min incubation time of the antioxidant solution to be tested with erythrocytes before analysis of the antioxidant protection capacity. The protective effect of the antioxidant was measured by assessing the area under the absorbance decay curve (AUC) of the sample. The result was compared with that of the phosphate buffer solution (PBS), which had received no antioxidant compound with AAPH as a blank. Quercetin was used as a standard to create a calibration curve, which was performed in duplicate. Each sample was analyzed in sextuplicate. Only blood samples between 30 and 48 days after collection were used. In Costa Rican blood banks, blood cannot be used for transfusion after a maximum of 30 days of storage and is thus discarded.
Fruit extracts were diluted with PBS to obtain a net area under curve (net AUC sample) within the linear response area of the net AUC of the quercetin standard. This information was determined by preliminary assays, and the dilution factors were as follows: 400 for blackberry, blueberry, coffee cherry, starfruit, tamarind, and mamey, 200 for cashew apple, dragon fruit, all the guavas and noni, 100 for lime and yellow pitahaya, 50 for ice cream bean fruit, kiwi, orange, peach palm, soursop, Surinam cherry and tree tomato, 20 for granadilla, papaya, pineapple, rambutan and watermelon, and 10 for all the other fruits.
The ERYCA values were expressed in micromoles of QE per 100 g of edible fresh fruit. The possible toxicity and good stability of all fruit extract / erythrocyte systems were always checked using the same procedure with the PBS-erythrocyte solution but with no AAPH. For all fruit extracts, the baseline absorbance remained constant at A =700 nm 1 ± 0.2 throughout the entire assay (380 min).
Hydrophilic oxygen radical absorbance capacity assay (H-ORAC)
The efficacy of fruit extracts to scavenge peroxyl radicals was measured using the H-ORAC assay according to the protocol as described in other studies [25,26].
Nonetheless, to allow comparison with ERYCA value and assess the cell-mediated antioxidant protection (Cell-MAP), H-ORAC values were reported in micromoles of quercetin equivalents (QE). The average H-ORAC value of 1 μM of quercetin is 5 ± 0.5 μM TE [20,27].
Cell-mediated antioxidant protection (Cell-MAP)
Cell-MAP of erythrocytes was evaluated by deducing the H-ORAC value of each fruit extract from the ERYCA value in QE. The results are expressed as the same quercetin equivalent standard (QE) per 100 g of edible fresh fruit.
Determining the total polyphenolic content
The total polyphenolic (TPP) content of fruit extracts was measured using the Folin–Ciocalteu method [28], which has recently been modified [29] to prevent interference from non-phenolic compounds. Because the Folin–Ciocalteu reagent is not sufficiently specific to phenolicics and can react with non-phenolic compounds, such as amino acids, and reduce sugars and vitamin C [29], the procedure was performed before and after solid-state extraction. This method utilized an OASIS® HLB 6cc extraction cartridge (Waters Corporation, Milford, MA). The eluate, which contained interfering water-soluble components, was recovered after the fruit extract (2 ml) had settled on the resin cartridge and was thoroughly washed with 2 ml of distilled water.
To measure absorbance, 2.5 ml of Folin–Ciocalteu reagent was added to 0.5 ml of the initial fruit extract or eluate. After 2 min, 2 ml of a 7.5% sodium carbonate solution was added, and the sample was immediately incubated in water bath at 50°C for 15 min for color development. Absorbance was read at 760 nm using a UV-1700 spectrophotometer (Shimadzu Corporation, Tokyo, Japan). On the basis of a standard curve obtained for different concentrations of gallic acid, the results were expressed as gallic acid equivalents (GAE). The value in GAE of interfering non-phenolic water-soluble components was calculated from the absorbance obtained for the eluate (Ve). The value for total polyphenolic content (TPP) was obtained by deducing the interference value (Ve) from the initial value obtained for fruit extract before elution (Vi) (i.e., TPP=Vi-Ve). All measurements were performed independently and in triplicate on two different samples, and the results were expressed as milligrams of gallic acid equivalents (GAE) per 100 g FW of edible fruit parts.
Statistical analysis
All statistical analyses were performed using XLSTAT software (Addinsoft SARL, Paris, France). Student’s t-test was used to compare the means of two samples, and the statistical level significance (P-value) was calculated. Pearson coefficients were calculated to assess the correlation between the two sets of data.
The ERYCA and H-ORAC methods are very similar except that in the first assay, human erythrocyte cells were used instead of the fluorescein used in the H-ORAC assay. Figure 1 presents an example of the type of curves obtained in the ERYCA assay during the In Vitro assessment of an antioxidant compound’s capacity to protect human erythrocytes from oxidative stress induced by free radicals released by AAPH. As in the H-ORAC assay, the protective capacity of an antioxidant is determined from the net area under a decay curve. It was observed that the natural antioxidant defense system of erythrocytes provides good protection for at least 1 hour after the induction of oxidative stress (absorbance at 700 nm remained almost stable). However, absorbance decreased sharply thereafter, which indicates cell disintegration and hemolysis. Like all other living cells, human erythrocytes have a Cell-MAP system, which includes a plasma membrane redox system (PMRS) and an antioxidative enzymatic system. Such a system provides a cell with an extra level of defense against extracellular oxidants and enables the cell to respond to changes in both the intra and extracellular oxidative environment [10,15,21,23]. The PMRS transfers electrons from intracellular donors to extracellular receptors to maintain a reduced surrounding environment [21-23], whereas erythrocyte enzymes, such as glutathione peroxydase superoxide dismutase, and catalase activities, among others, work to reduce organic peroxides [11]. Nonetheless, after one hour in our test conditions, the constant attack of free radicals overwhelmed the Cell MAP system without the help of exogenous antioxidant compounds. This condition was indicated by the rapid loss of the erythrocytes’ light-scattering properties. When a fruit extract or quercetin was added to the medium, the resistance of erythrocytes to oxidative stress was significantly enhanced. The decrease in absorbance was hindered proportional to the concentration of antioxidant compound or fruit extract and their respective protective capacity (Figure 1 and 2). It was checked that the net area under the curves that corresponded to suspended erythrocytes with only AAPH, or to erythrocytes with AAPH and an antioxidant compound (quercetin) or fruit extract (blackberry and papaya), were proportional (r2>0.97 in all cases) to the concentrations of the standard or fruit extracts (Figure 2). For quercetin, the chosen standard, the highest correlation of net area under the curve and QE concentration was checked between 2 and 24 mm and this range was used for all assays. Quercetin was chosen as the standard because this molecule is regularly found in the bloodstream after ingestion of phenol-rich foods at plasma concentrations ranging from 0.3-0.75 μm [30], which is not very far from the range of concentration tested in the ERYCA assay. In agreement with previously published results, the correlation coefficient r2 between the net area and quercetin concentration was always above 0.98, independent of blood donors and storage time (up to 50 days) [20]. Additionally, even though the antioxidant defense systems in erythrocytes is depleted during blood storage [11] and can vary with donor age and storage time, the inclusion and use of quercetin calibration curves in each ERYCA assay achieves repeatable results, which limits inter-assay variability (Table 1) [20]. Consequently, the net area under the curve obtained for a specific diluted fruit extract can be related to an equivalent concentration of QE per mass (100 g FW) of edible fruit parts analyzed.
| ERYCA value without incubation | ERYCA value after 15 min incubation | ||||||
|---|---|---|---|---|---|---|---|
| mean | Conf. intervala | % | mean | Conf. intervala | % | ||
| Antioxidant | (µMole QE/ µMole of standard) | (µMole QE/µMole of standard) | |||||
| Quercetin | 1.07 | 0.13 | 12.1% | 1.04 | 0.07 | 5.8% | |
| Ascorbic acid | 0.18 | 0.03 | 16.7% | 0.12 | 0.01 | 10.4% | |
| Tannic acid | 6.12 | 1.21 | 19.7% | 4.58 | 0.20 | 4.5% | |
| Catechin | 1.87 | 0.17 | 9 % | 1.71 | 0.037 | 2.2 % | |
| Trolox | 0.46 | 0.06 | 13% | 0.40 | 0.02 | 5.9% | |
| Fruit extract | (µMole QE. g-1 DM) | (µMole QE. g-1 DM) | |||||
| Blackberrya | 352 | 33.5 | 9.5% | 390 | 15.6 | 4 % | |
| Noni | 80 | 1.25 | 1.6% | 84 | 1.5 | 1.8% | |
aindicates variation with respect to mean of confident interval at 95 % ( 1.96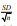
Table 1: Reproducibility and accuracy of the ERYCA test for different antioxidant standards and two tropical fruit juices with a 15-min incubation and no incubation previous to the test.
Because the interaction of antioxidant compounds and red blood cells should be expected to allow better diffusion through the medium, and eventually through erythrocyte membranes, the introduction of 15-min incubation times of antioxidant solution with erythrocytes previous to the assessment of the antioxidant protection capacity significantly reduces the standard deviation around the mean (Table 1) when compared with assays that had no preceding incubation times. However, both means remained statistically similar (P-value<0.05). It has been shown that quercetin [23], after only 30 minutes of incubation, can accumulate within erythrocyte membranes in much higher concentrations compared to the surrounded fluid media. Other studies have shown similar results for plant flavonoids that were located within the phospholipid bilayer of the erythrocyte membrane after one hour of incubation [15].
The improved ERYCA method was used to assess the erythrocyte cellular antioxidant activity of hydro-acetone extracts from 34 tropical fruits widely consumed in tropical Latin America. The H-ORAC antioxidant capacity of the same extracts was also measured, and the results are shown in Table 2, the results are presented as QE. Figure 3 presents the fruits classified according to their Cell-MAP effect on erythrocytes. Cell-MAP values were obtained by deducing, from the ERYCA value, the protection capacity induced by the direct scavenging of free radicals in the surrounding medium, as assessed by the H-ORAC assay. Figure 4 presents the values of phenolics content obtained on fruit extracts (Vi) using the modified Folin–Ciocalteu method (in GAE) because these data are commonly reported in the literature and the values correspond to the interference of non-phenolic compounds.
| Fruit name | Taxonomic name | Moisture (%) |
ERYCAa,b (mmol QE.100 g-1 FW) |
H-ORACa,b (mmol QE.100 g-1 FW) |
Cell-MAP (mmol QE.100 g-1 FW) |
% Cell-MAP |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Avocado | Persea americanavar. Hass | 71.6 | 124 | ± | 6 | 43 | ± | 4 | 81 | ± | 7 | 65% |
| Banana | Musa AAA cv. Grand nain | 86.2 | 398 | ± | 21 | 112 | ± | 5 | 286 | ± | 22 | 72% |
| Blackberry (tropical highland) | Rubus adenotrichus | 85.4 | 5757 | ± | 358 | 1865 | ± | 124 | 3892 | ± | 379 | 68% |
| Blueberry (Costarican) | Vaccinium consanguineum | 76.5 | 6345 | ± | 395 | 2043 | ± | 101 | 4302 | ± | 408 | 68% |
| Cashew apple | Anacardium occidentale | 88.2 | 3796 | ± | 180 | 436 | ± | 22 | 3360 | ± | 181 | 89% |
| Chayote (cooked) | Sechium edule | 93.9 | 69 | ± | 5 | 5 | ± | 0 | 64 | ± | 5 | 93% |
| Coconut water | Cocos nucifera | 96.2 | 1 | ± | 0.5 | 0 | ± | 0 | 0,08 | ± | 0,09 | 80% |
| Coffee cherry | Coffea arabica | 89.2 | 601 | ± | 20 | 288 | ± | 13 | 313 | ± | 24 | 52% |
| Costarican guava | Psidium friedrichsthalianum | 85.4 | 2542 | ± | 119 | 456 | ± | 7 | 2086 | ± | 119 | 82% |
| Dragon fruit (red Pitahaya) | Hylocereus costaricensis | 89.1 | 1738 | ± | 64 | 187 | ± | 11 | 1551 | ± | 65 | 89% |
| Granadilla | Passiflora ligularis | 83.1 | 612 | ± | 18 | 51 | ± | 3 | 561 | ± | 18 | 92% |
| Guava (Pink flesh) | Psidium guajava | 86.3 | 2777 | ± | 117 | 522 | ± | 35 | 2255 | ± | 122 | 81% |
| Guava (white flesh) | Psidium guajava | 85.9 | 2351 | ± | 93 | 515 | ± | 34 | 1836 | ± | 99 | 78% |
| Kiwifruit | Actinidia deliciosa | 86.6 | 287 | ± | 22 | 122 | ± | 8 | 165 | ± | 23 | 58% |
| Lime (Rangpur) | Citrus × limonia Osbeck | 91.5 | 328 | ± | 13 | 68 | ± | 3 | 260 | ± | 13 | 79% |
| Mamey | Pouteria sapota | 81.3 | 3764 | ± | 183 | 966 | ± | 47 | 2798 | ± | 189 | 74% |
| Mango | Mangifera indica var. criollo | 84.2 | 380 | ± | 16 | 51 | ± | 2 | 329 | ± | 16 | 87% |
| Melon | Cucumis melo var. cantaloupe | 90.6 | 246 | ± | 4 | 62 | ± | 3 | 184 | ± | 5 | 75% |
| Noni | Morinda citrifolia | 86.8 | 1421 | ± | 31 | 270 | ± | 10 | 1151 | ± | 33 | 81% |
| Orange | Citrus sinensis var. valencia | 87.1 | 580 | ± | 22 | 177 | ± | 10 | 403 | ± | 24 | 70% |
| Papaya | Carica papaya | 86.3 | 168 | ± | 12 | 147 | ± | 7 | 21 | ± | 14 | 13% |
| Passion fruit (yellow) | Passiflora edulis var. flavicarpa | 85.7 | 907 | ± | 33 | 93 | ± | 4 | 814 | ± | 33 | 90% |
| Peach Palm fruit (boiled) | Bactris gasipaes | 57.6 | 30 | ± | 2 | 10 | ± | 0 | 20 | ± | 2 | 67% |
| Pineapple | Ananas comosus var. golden | 84.5 | 279 | ± | 18 | 146 | ± | 5 | 133 | ± | 19 | 48% |
| Pitahaya Yellow | Hylocereus megalanthus | 87.6 | 1355 | ± | 52 | 257 | ± | 16 | 1098 | ± | 54 | 81% |
| Plantain (ripe fried) | Musa paradisiaca L AAB | 58.4 | 120 | ± | 7 | 32 | ± | 1 | 88 | ± | 7 | 73% |
| Plantain (unripe boiled) | Musa paradisiaca L AAB | 64.8 | 87 | ± | 3 | 18 | ± | 1 | 69 | ± | 3 | 79% |
| Rambutan | Nephelium lappaceum | 85.1 | 98 | ± | 4 | 16 | ± | 1 | 82 | ± | 4 | 84% |
| Soursop | Annona muricata | 85.0 | 249 | 16 | 152 | ± | 10 | 97 | ± | 19 | 39% | |
| Star fruit | Averrhoa carambola | 92.3 | 1820 | ± | 81 | 539 | ± | 21 | 1281 | ± | 84 | 70% |
| Surinam cherry | Eugenia uniflora | 88.6 | 418 | ± | 22 | 145 | ± | 11 | 273 | ± | 24 | 65% |
| Tamarind | Tamarindus indica | 56.1 | 1209 | ± | 25 | 373 | ± | 28 | 836 | ± | 38 | 69% |
| Tree tomato | Solanum betacea | 84.5 | 732 | ± | 53 | 254 | ± | 16 | 478 | ± | 55 | 65% |
| Watermelon | Citrullus lanatus | 92.2 | 66 | ± | 3 | 45 | ± | 2 | 21 | ± | 3 | 32% |
| average | 1225 | ± | 109 | 208 | ± | 17 | 917 | ± | 62 | 71% | ||
a Data are expressed as fresh weight (FW) of total edible portion of the fruit as the usual form of consumption.
bAverage value of 2 samples analyzed independently each in sextuplicate ± confidence interval at 95%
Table 2: ERYCA, H-ORAC of 34 common fruits of tropical America.
In Figure 3 and Table 2, of the fruits tested, two Costa Rican berries had the highest scores for ERYCA, H-ORAC, Cell-MAP and TPP.
If we compare the global cellular antioxidant capacity expressed by ERYCA values and H-ORAC values according to the same QE standard, both tropical highland berries provide erythrocytes with approximately 3 times more protection against oxidative stress than fluorescein, as assessed by the H-ORAC method. In this case, the antioxidant capacity of fruit extracts is considerably enhanced by erythrocyte presence, and Cell-MAP represents 68% of global protection to erythrocytes, as assessed by the ERYCA assay, whereas only 32% is due to the direct scavenging of free radicals in the surrounding medium, as assessed by the H-ORAC assay. Recent findings suggest that anthocyanins can interact with erythrocytes because they seem to play an important role in stabilizing red blood cell membranes [31], which also suggests higher cell-related antioxidant protection. When compared with H-ORAC, the difference with the ERYCA value indicates a level of protection beyond the simple scavenging effect of free radical in the surrounding solution, as assessed by H-ORAC method.
This observation was true for most of the fruits studied, illustrated by the fact that the erythrocyte protection capacity assessed by the ERYCA method (expressed as μmol QE per 100 g FW) is significantly different (P-value < 0.02 for t-test for paired H ORAC/ERYCA mean comparison) and 3.3 times more, on average than the protection capacity measured for fluorescein, as assessed by the H-ORAC method (Table 2). Consequently, approximately 70% of the global protective effect of all fruit extracts, on average, is due to synergistic effects between the natural defense system of erythrocytes and natural compounds present in fruit extracts. In the case of berries, Cell-MAP is relatively low when compared to the other fruits studied for which the extra level of protection due to the presence of cells represents around 90% (Table 2). Such is the case for cashew apple (Anacardium occidentale L.) and mamey (Pouteria sapota), which scored the third highest global cellular antioxidant protection capacity, as assessed by the ERYCA assay. Specifically, the main phenolic compound of cashew apple is the anacardic acid [32,33], which is a phenolic lipid that interacts with the phospholipidic bilayer of erythrocytes [34,35]. From there, it could act as an intracellular substrate of PMRS oxidoreductase, as in the case of quercetin, and enhance membrane protection against free radicals [10,21,24].
Interestingly, all guava species (pink- and white-flesh varieties of Psidium guajava L. and the Costa Rican guava Psidium friedrichsthalianum (O.Berg) Nied.) scored high values with the ERYCA method (Figure 3). On average, ERYCA values were 5 to 6 times more than for the H-ORAC method when compared to the same quercetin equivalent standard, which indicates high Cell-MAP effects approximately 80% of global cellular antioxidant capacity.
Starfruit (Averrhoa carambola L.) and dragon fruit (Hylocereus costaricensis (F. A. C. Weber) Britton & Rose, also known as red pitahaya) had the next highest ERYCA and Cell-MAP scores. Noni (Morinda citrifolia L.), yellow pitahaya (Hylocereus megalanthus (K. Schumann ex Vaupel) Ralf Bauer), tamarind (Tamarindus indica L.), and passion fruit (Passiflora edulis Sims var. flavicarpa) formed a group with relatively high ERYCA and Cell-MAP scores. Within this group, the Cell-MAP of passion fruit extract presented almost 90% of global cellular antioxidant capacity as assessed by ERYCA method, although its TPP value is relatively low when excluding interferences. As with dragon fruit, yellow pitahaya and noni extracts provided high Cell- MAP. Because they all presented somewhat low amounts of phenolics (TPP < 65 mg GAE per 100 g FW) and relatively little direct peroxylradical- scavenging activity, as assessed by the H-ORAC method, enhanced Cell-MAP could be attributed to specific compounds with a significant ability to interact with erythrocyte cells. Nonetheless, this poorly studied group of fruits requires a more detailed study of their chemical composition to determine which antioxidant compounds may be able to interact with red blood cells and enhance membrane protection.
Another fruit groups with average Cell-MAP values contained are: granadilla, tree tomato, orange, mango, coffee cherry, banana Surinam cherry, lime, and melon. The last fruit group had low scores for ERYCA, Cell-MAP and H-ORAC tests, including kiwifruit, pineapple, soursop, plantain, rambutan, avocado, chayote, papaya watermelon, green coconut water, and cooked tropical fruits (plantain, chayote, and peach palm). Except for rambutan, all these fruits also had very low phenolic contents (Figure 4), which may explain these low scores.
Within this group, cooked fruits (plantain and peach-palm fruit) have very low antioxidant activity. These strarchy fruits are generally consumed cooked, and the cooking process could reduce the antioxidant content. Although peach-palm fruit is an important source of carotenoids, mainly carotene isomers [36], the extract has a relatively low ERYCA value. Nonetheless, it is slightly higher than H-ORAC when expressed using the same standard. Fried ripe plantain shows slightly higher antioxidant values than unripe boiled plantain in both the ERYCA and H-ORAC assays and TPP, which is most likely due to higher dry matter content.
Overall, Pearson correlations between ERYCA, H-ORAC, Cell- MAP and TPP were all positive, with r ranging between 0.830 and 0.939 on either a fresh or dry weight basis (Table 3). These high correlations were not observed in previous work [20] because much fewer data were processed. Even so, these overall correlations hide discrepancies between the methods, as previously observed. Note that the correlation coefficient is much lower for the value (Vi), the common value reported in the literature for total phenolic content, which is given when the Folin–Ciocalteu method is used directly on the initial fruit extract without eliminating interference from non-phenolic compounds.
| Variables | ERYCA | H-ORAC | Cell-MAP | TPP | Vi |
|---|---|---|---|---|---|
| H-ORAC | 0.939 (0.904a) | ||||
| Cell-MAP | 0.991 (0.989) | 0.884 (0.830) | |||
| TPP | 0.916 (0.885a) | 0.900 (0.853a) | 0.893 (0.856) | ||
| Vi | 0.836 (0.746a) | 0.823 (0.728a) | 0.814 (0,719) | 0.958 (0.928a) | |
| Ve | 0.110 (0.108a) | 0.117 (0.127a) | 0.103 (0.097) | 0.272 (0.337a) | 0.538 (0.664a) |
a indicates correlation on dry weight basis
Table 3: Pearson Correlation coefficients for ERYCA, H-ORAC, TPP, Vi and Ve.
These results suggest that it would be useful to determine the relative participation of fruits within the overall antioxidant capacity potential of the usual diet consumed in tropical America. For millions of people in this region, which encompasses Central America, the Caribbean Region and northern South America, meals are centered on rice and beans and often accompanied by cooked plantain, a fruit juice, and the occasional portion of meat.
White rice once cooked presents undetectable ERYCA values and phenolics content. Black beans (Phaseolus vulgaris L.) cooked as usual present an ERYCA value of 9.3 ± 0.3 μmol QE per 100 g FW and a TPP value of 43.6 ± 1.7 g GAE per 100 g. Two juices consumed as part of a usual tropical American diet (Figure 5) were used for comparison: one was made with papaya, which has a relatively low ERYCA score; and the other was made with blackberry juice, which has one of the highest ERYCA scores. The contribution of the total antioxidant potential, as assessed by ERYCA assays for all fruit-based products, (plantain + fruit juice) is between 95% for papaya juice and 99% with blackberry juice. These results indicate that fruit-based products are an important potential source of antioxidants in the diet, particularly in Tropical Latin-America. This information is highly relevant because fruit is consumed by millions of people, including the poorest, who tend to replace the traditional habit of consuming fruit juices with soda drinks, which have very low or no antioxidant capacity.
aPapaya mixed with ½ water (w/w) bTropical highland blackberry mixed with water (1/10) (w/w)
Figure 5: Respective contribution of global cellular antioxidant potential assessed by the ERYCA assay of a common meal composed by cooked rice (100 g), beans (150 g) and plantain (80 g) with 250 ml of papaya or tropical highland blackberry juice.
Almost all the tropical fruit extracts tested presented much higher protection to erythrocytes than to fluorescein alone, which indicates the high relative importance of Cell-MAP in the global antioxidant mechanism. In fact, even if fruit compounds exert direct free-radical scavenging effects (as assessed by most antioxidant screening methods, including H-ORAC), it is the synergistic effects of these compounds with the endogenous antioxidant systems of cells (in this study, the erythrocytes) that are the most important in their global protective mechanism. This Cell-MAP effect represents, in most cases, between 70 and 90% of global antioxidant protection, which can be easily deduced from the comparison of the two assays, H-ORAC and ERYCA, performed under similar conditions. Cell-MAP can be used to screen compounds with the ability to enhance the endogenous antioxidant protection system of cells. Although we should be extremely cautious in speculating about the health consequences of these fruits, the high Cell-MAP value indicates a potential positive biological impact, at least at the level of the gastro-intestinal tract where human epithelial cells may be in direct contact with food components. Nonetheless, further speculation on possible systemic health effects cannot be based only on these results as In Vitro assays performed on food extracts do not take into account the impact of molecule bioavailability and digestion, including bioconversion of nutrients by microbial flora. In Vitro assays directly performed on food extracts are useful for screening potentially interesting food extracts and molecules, but a second step is required to more deeply explore bioavailability issues.
The authors wish to thank Marielos Torres, Paula Valverde, and Randall Cordero for their technical assistance.