Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2016) Volume 6, Issue 1
The study aims to find out best treatment to increase shelf life of rice bran oil. Gas-Liquid Chromatography was, therefore, used to characterize essential and non-essential fatty acid profiling in oils extracted from rice brans treated with Phosphoric acid (1.0 and 1.5%), Acetic acid (5 and 7%), Hydrochloric acid (20 and 30 ml/kg of rice bran), Sodium metabisulphite (1.0 and 1.5%), rice bran oils extracted from rice treated at 100°C for one minute and steamed at 100°C for 20 minute. Results suggested that both the essential fatty acids i.e., linolenic acid (C18:3) and linoleic acid (C18:2) constitutes 1.47% and 31.34% of the total fatty acids in all the rice bran oil samples. Rice bran oil therefore can be utilized as a good source of linoleic (C18:2) an essential fatty acid. Oleic (C18:1), linoleic (C18:2) and palmitic (C16:0) acids were dominant fatty acids in all the parboiled (48.53, 27.83 and 18.03 respectively) and un-parboiled (43.39, 41.34 and 16.54% respectively) rice samples. The total content of fatty acid (ΣFFA) such as mystiric acid, palmitic acid, oleic acid, ficosanoic acid, of parboiled bran oil was found higher than that of unparboiled rice bran oil samples. Whereas, other fatty acids were present in higher quantities in bran oils of unparboiled. The ratio of unsaturated fatty acids of each rice bran oil sample was much higher (~80%) than its ratio of saturated fatty acid (~20%). Results also indicated that parboiling increases the shelf life of bran oil by lowering proportions of unsaturated fatty acids and increasing saturated free fatty acids contents in rice bran oil. Results suggests that steaming and extrusion methods can be used as a good alternatives to harmful chemicals being used to make rice bran oils more stable for longer period of time.
Keywords: Rice bran oil; Essential and non-essential fatty acids; Gas liquid chromatography; Parboiling; Chemical treatments; Extrusion method
PT: Parboiled Treatments
RT: Raw rice Treatments
Rice bran, being co-product obtained after milling of brown rice, remains unexploited annually in abundant quantities, is actually an incomparable source of minerals and vitamins that is wasted as such. Research conducted during the last two decades showed that rice bran is a unique complex of naturally occurring antioxidant compounds [1]. It constitutes 10% of the total rough rice grain in terms of weight [2]. More than 500 million metric tons of milled rice is produced per year all over the world, constituting more than a quarter of all cereal grains [3-4]. People of Asia, South America, mush of Africa, and parts of Europe, the near East and North America depend upon rice for daily sustenance [5]. Collectively, it can be summarized that 5 million metric tons of rice portion (pericarp, aleurone, sub-aleurone, seed coat, nucellus along with the germ and small proportions of endosperm) is being discarded annually in the form of rice bran.
In Pakistan, milled rice (Oryzae sativa) is commonly consumed country-wide, at household level where it is boiled before consumption. However, at industrial level, parboiled processed rice is used. More than 6 million metric tons of milled rice is produced every year in Pakistan [4]. Parboiled rice, sometimes also called as converted rice, is partially boiled un-husk rice. The three basic steps of parboiling are soaking, steaming and drying [6]. Parboiling drives nutrients, thiamine, from the bran to endosperm [7], hence parboiled white rice is 80% nutritionally similar to brown rice and more nutritional than white milled rice. Because of this, parboiling is now being adopted by more than 80% countries of the world. Therefore, the bran produced during milling of parboiled rice, is chemically dissimilar to unparboiled rice.
Keeping in above mentioned view, it is a dire need to carry out the fatty acid profiling of the rice bran oil, so as to determine its edibility in terms of fatty acid consumption as well as to increase its shelf life using various chemical treatments on rice bran oil samples. Rice bran is considered an excellent source of rice oil. Fatty acids may be divided into essential and no-essential types. Essential fatty acids are defined as those that cannot be synthesized de novo in sufficient quantities for normal physiological function of human body. Collins et al. [8] were the first to demonstrate a linoleic acid deficiency in adults. They reported that patients undergoing intravenous nutrition with glucose became isolated from their fat supplies and speedily developed biochemical signs of essential fatty acid deficiency (an increase in 20:3/20:4 ratios in plasma) and skin symptoms. This could be treated by infusing lipids [9]. Linoleic acid has a specific role in maintaining the skin water-permeability barrier, probably as constituents of acylglycosylceramides. Therefore, diet having a sufficient quantity of essential fatty acids is considered significant in everyday food. On the basis of above discussion, main objective of this study was also to find out proportions of different types of essential and non-essential fatty acids present in oils extracted from rice bran.
Shelf Life Index (SLI) or sometimes also called as Oil Stability Index (OSI), is an American Oil Chemists Society (AOCS) approved method that determines the relative resistance of fat and oil samples to oxidation. All fats and oils are prone to oxidation. The rapidity of oxidation depends on the degree of unsaturation, the presence of antioxidants, and prior storage conditions. Knowing the percentages of saturated and unsaturated free fatty acids in an oil sample using free fatty acid analyses which give an idea of how good or bad oil is at a particular time, the SLI can be measured. SLI can be used to compare various oils to predict their respective shelf lives. The SLI value helps to evaluate the effectiveness of antioxidants or determine how much longer frying oil can be used before it goes bad (Figure 1).
Conventional methods for the determination of free fatty acids [10] provide less information and wrong estimates of fatty acid contents. In this research work, free fatty acids were determined by using GL chromatography for more precise measurement fatty acid contents and acid heptadecanoic (C17:0) was used as an internal standard. This technique has already been used by other researchers for fatty acid profiling in different research experiments such as Zhout et al. [11], Shehzad et al. [12] and Tead et al. [13].
The study was conducted in rice technology lab of Rice Research Institute (RRI), Kala Shah Kaku, and Lahore, Pakistan. Rice samples were obtained from bulks of Basmati 515, a newly approved variety, most suitable for parboiling, harvested in first week of November, 2012. Samples were stored in cool and dry place in sealed glass jars.
Parboiling treatment
After three days of soaking, one half of the bulk was undergone parboiling process that included two drying cycles. The drying temperature during 1st pass is 95°C and during 2nd pass was 75°C. The steamed paddy was dried at a temperature of 95°C during first pass, below the starch gelatinization Temperatures, till moisture content of treated paddy reaches 18%. After 1st pass, the partially dried paddy was tempered at room temperature for a minimum period of 2-3 hours. Presoaking below the gelatinization Temperatures minimizes the splitting of grains. After tempering, drying temperature during the second drying cycle was 75°C, till the treated paddy reaches 11% moisture content. The treated and dried paddy samples were milled.
Chemicals and glassware
Chemicals such as Phosphoric acid (1.0%, 1.5%), Acetic acid (5.0%, 7.0%), Hydrochloric acid (5.0%, 7.0%), Sodium meta-bi-sulphite (1.0%. 1.5%) were analytical grade from E. Merck. All the glassware used in the experiment, were thoroughly washed with liquid soap, then rinsed and soaked in a mixture of distilled water (dH2O) and 5% HNO3 for one day (24 hours) in order to remove other impurities. They were then drained after washing.
Collection of rice bran
Parboiled and un-parboiled samples of rice were cleaned with a seed blower. 1 kg of each treated and raw dried (less than 12% moisture content) paddy samples were hulled with a testing husker (THU, 35H, Satake Engineering Co. Ltd., Japan). The moisture content of each sample was predetermined using a Steinlite Model 500 RC Electronic moisture tester. The samples were then whitened in a single pass friction rice pearler (BS08A, Satake Engineering Co. Ltd., Japan) with the degree of whiteness set between ‘Low’ and ‘Medium’ on the equipment. After milling, rice bran was removed with a 1.7 mm sieve and collected separately in glass jars. These jars were sealed and stored in cool dry place.
Extraction of rice bran oil
Extraction of oil from rice bran samples was done by solvent extraction method using a Laboratory Scale Rice Bran Oil Extraction Unit.
Rice bran stabilization
The processing of rice bran was carried out immediately to inactivate endogenous lipases, responsible for fat deterioration. To achieve this objective, rice bran was subjected to extrusion stabilization technique.
Extrusion stabilized rice bran
Extrusion heating rice bran was prepared using a locally made Extrusion Rice Bran Stabilizer with 80 Kg/hr production rate. The Raw and Parboiled was fed directly into the extruder with 12-13% moisture contents. The extruder is operated at 99-100°C for 30 sec, held for 1 min at 99°C to inactivate lipase and dried in hot air dryer at 80°C and cooled at room temperature [14]. The temperature of 100°C is sufficient to inactivate the lipases completely in the raw as well as parboiled rice bran [9]. At last the samples were then packed in food graded polyethylene bags to prevent moisture absorption [10].
Steaming method
10 g of parboiled and un-parboiled samples were subjected to steaming 100°C for 20 minutes without extrusion. The samples were then packed in food graded polyethylene bags to prevent moisture absorption [10].
Chemical treatments
1 g oil extracted from parboiled and un-parboiled sample was treated with different chemicals such as Phosphoric acid (1.0%, 1.5%), Acetic acid (5.0%, 7.0%), Hydrochloric acid (5.0%, 7.0%), and Sodium metabisulphite (1.0%, 1.5%) and subjected to GLC for fatty acid profiling.
Fatty acid profile analysis
Fatty acids need to be converted into fatty acid methyl esters (FAME) for GLC analysis [11]. For the purpose, 15 g of each oil sample was poured into a test tube and 2 ml 5M KOH was added. The solution was heated at 85°C for 10 min. The resulting solution was then neutralized with 0.7M HCL. The resulted solution was further heated at same temperature for 10 minutes and methylated oil was extracted with petroleum ether. The fatty acid methyl esters were then separated and subjected to GLC with RESTEK 100 M MXT-1 (100 m, 0.25 mm ID, 0.2 μm columns). Helium was the carrier gas at the flow rate of 65 psi and a syringe injection. The temperature was programmed according to the specifications mentioned by IUPAC, 1987. Identification and quantification of the methyl esters were made by comparison of retention time with standard fatty acid methyl esters.
Shelf life index (SLI) was calculated by the following formula also used by Zhout et al. [11]:
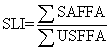
ΣSAFFA = Total saturated free fatty acid
ΣUSFFA = Total unsaturated free fatty acid
The mass spectrum of new peak was compared with that of standard for fatty acid identification. Oleic (C18:1), linoleic (C18:2) and palmitic (C16:0) acids were dominant fatty acids in all the parboiled and unparboiled samples [15]. These results are also in agreement with Zhout, et al. [11] while investigating fatty acid composition of three rice varieties after different storage durations. The total content of fatty acid (ΣFFA) such as mystiric acid, palmitic acid, oleic acid, ficosanoic acid, of parboiled bran oil was found higher than that of un-parboiled rice bran oil samples. While the other fatty acids were found less in bran oils of differently treated parboiled rice as compared un-parboiled samples. Tables 1 and 2 show the free fatty acid (FFA) compositions of differently treated raw and parboiled rice bran oils respectively. Statistical analysis showed significant differences (P ≤ 0.05) in fatty acid composition of oil undergone all the treatments (PT1-PT10 for parboiled and RT1-RT10 for raw rice bran oils) practiced on parboiling as well as un-parboiled samples respectively.
| Treatments | Fatty acid (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C14:1 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2* | C18:3* | C20:0 | C20:1 | C22:0 | ∑SAFFA | ∑USFFA | SLI | |
| Phosphoric acid @ 1.0% (RT1) | 0.331 | 0.107 | 17.259 | 0.577 | 1.946 | 44.175 | 31.418 | 1.499 | 0.674 | 1.619 | 0.396 | 20.27 | 79.4 | 0.255 |
| Phosphoric acid @ 1.5% (RT2) | 0.325 | 0.101 | 17.274 | 0.572 | 1.914 | 44.097 | 31.527 | 1.507 | 0.669 | 1.609 | 0.406 | 20.26 | 79.41 | 0.255 |
| Acetic acid @ 5.0% (RT3) | 0.33 | 0.106 | 17.235 | 0.576 | 2.081 | 44.114 | 31.375 | 1.498 | 0.673 | 1.618 | 0.394 | 20.38 | 79.29 | 0.257 |
| Acetic acid @ 7.0% (RT4) | 0.281 | 0.089 | 16.476 | 0.505 | 1.924 | 41.405 | 30.224 | 1.336 | 6.005 | 1.541 | 0.215 | 24.62 | 75.1 | 0.327 |
| Hydrochloric acid @ 20.0ml/kg (RT5) | 0.331 | 0.115 | 8.572 | 0.555 | 2.149 | 42.729 | 31.315 | 1.422 | 0.668 | 1.631 | 0.511 | 11.9 | 77.77 | 0.153 |
| Hydrochloric acid @ 30.0ml/kg (RT6) | 0.308 | 0.098 | 18.691 | 0.538 | 2.244 | 43.58 | 30.354 | 1.466 | 0.664 | 1.616 | 0.441 | 22.04 | 77.65 | 0.283 |
| Sodium metabisulphite @ 1.0% (RT7) | 0.328 | 0.106 | 17.725 | 0.588 | 1.928 | 42.989 | 31.888 | 1.552 | 0.679 | 1.606 | 0.612 | 20.94 | 78.73 | 0.266 |
| Sodium metabisulphite @ 1.5% (RT8) | 0.323 | 0.1 | 17.187 | 0.569 | 2.045 | 43.091 | 32.319 | 1.519 | 0.668 | 1.626 | 0.555 | 20.45 | 79.22 | 0.258 |
| Extrusion method @ 100oC, 1 min. (RT9) | 0.332 | 0.108 | 17.707 | 0.579 | 2.092 | 44.096 | 30.982 | 1.469 | 0.66 | 1.606 | 0.368 | 20.83 | 78.84 | 0.264 |
| Steaming method @ 100oC, 20 min. (RT10) | 0.32 | 0.115 | 17.25 | 0.572 | 1.972 | 43.581 | 31.945 | 1.452 | 0.705 | 1.62 | 0.469 | 20.4 | 79.28 | 0.257 |
| Std. Error of Mean | 0.0049 | 0.0025 | 0.903 | 0.008 | 0.035 | 0.276 | 0.211 | 0.019 | 0.533 | 0.008 | 0.035 | |||
| Std. Deviation | 0.016 | 0.008 | 2.855 | 0.025 | 0.112 | 0.872 | 0.668 | 0.06 | 1.687 | 0.025 | 0.11 | |||
| Min.-Max. | 0.281-0.332 | 0.089-0.115 | 8.572-18.691 | 0.505-0.588 | 1.914-2.244 | 41.405-44.175 | 30.224-32.319 | 1.336-1.552 | 0.660-6.005 | 1.541-1.631 | 0.215-0.612 | 0.153-0.327 | ||
| ∑FFA/∑n | 0.321 | 0.104 | 16.538 | 0.563 | 2.029 | 43.386 | 31.335 | 1.472 | 1.206 | 1.609 | 0.437 | 20.21 | 78.47 | 0.258 |
| ∑FFA | 3.208 | 1.045 | 165.377 | 5.631 | 20.294 | 433.858 | 313.346 | 14.721 | 12.06 | 16.091 | 4.366 | 202.1 | 784.7 | 2.579 |
Table 1: Fatty acid profiling/ composition of variously treated bran oil extracted from raw/ un-parboiled rice samples. *Sign shows the essential fatty acids. ΣSAFFA = total saturated free fatty acids, ΣUSFFA = total unsaturated free fatty acids, ΣFFA = total free fatty acids, ΣFFA/Σn = average of total free fatty acids, SLI = Shelf life index, C14:0 = Myristic acid, C14:1 = Myristoleic acid, C16:0 = Palmitic acid, C16:1 = Palmitoleic acid, C18:0 = Stearic acid, C18:1 = Oleic acid, C18:2 = Linoleic acid, C18:3 = Linolenic acid, C20:0 = Arachidic acid, C20:1 = Eicosanoic acid, C22:0 = Behenic acid.
| Treatments | Fatty acid (%) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C14:0 | C14:1 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2* | C18:3* | C20:0 | C20:1 | C22:0 | ∑SAFFA | ∑USFFA | SLI | |
| Phosphoric acid @ 1.0% (PT1) | 0.345 | 0.082 | 17.857 | 0.459 | 1.503 | 49.083 | 28.421 | 0.728 | 1.153 | 1.562 | 0.18 | 20.69 | 80.34 | 0.258 |
| Phosphoric acid @ 1.5% (PT2) | 0.327 | 0.084 | 17.642 | 0.484 | 1.506 | 48.403 | 28.466 | 1.2 | 0.968 | 1.743 | 0.177 | 20.29 | 80.38 | 0.252 |
| Acetic acid @ 5.0% (PT3) | 0.331 | 0.08 | 18.403 | 0.469 | 1.466 | 49.149 | 27.166 | 1.146 | 0.924 | 1.696 | 0.169 | 20.96 | 79.71 | 0.263 |
| Acetic acid @ 7.0% (PT4) | 0.316 | 0.078 | 17.797 | 0.465 | 1.396 | 48.423 | 28.779 | 1.005 | 0.872 | 1.711 | 0.159 | 20.22 | 80.46 | 0.251 |
| Hydrochloric acid @ 20.0ml/kg (PT5) | 0.331 | 0.075 | 18.054 | 0.462 | 1.445 | 47.793 | 28.918 | 1.115 | 0.919 | 1.717 | 0.171 | 20.59 | 80.08 | 0.257 |
| Hydrochloric acid @ 30.0ml/kg (PT6) | 0.339 | 0.078 | 18.978 | 0.478 | 1.432 | 49.371 | 26.614 | 0.969 | 0.904 | 1.657 | 0.179 | 21.49 | 79.17 | 0.271 |
| Sodium metabisulphite @ 1.0% (PT7) | 0.353 | 0.077 | 18.894 | 0.349 | 1.746 | 48.431 | 26.189 | 0.959 | 1.012 | 1.881 | 0.107 | 21.76 | 77.89 | 0.279 |
| Sodium metabisulphite @ 1.5% (PT8) | 0.363 | 0.093 | 17.738 | 0.352 | 1.604 | 47.323 | 28.542 | 0.984 | 1.025 | 1.879 | 0.097 | 20.46 | 79.17 | 0.258 |
| Extrusion method @ 100oC, 1 min. (PT9) | 0.348 | 0.089 | 16.074 | 0.348 | 1.416 | 48.825 | 28.947 | 0.958 | 1.011 | 1.579 | 0.105 | 18.61 | 80.75 | 0.23 |
| Steaming method @ 100oC, 20 min. (PT10) | 0.354 | 0.086 | 18.948 | 0.35 | 1.454 | 48.571 | 26.264 | 0.962 | 1.015 | 1.484 | 0.11 | 21.53 | 77.72 | 0.277 |
| Std. Error of Mean | 0.005 | 0.002 | 0.274 | 0.019 | 0.033 | 0.197 | 0.359 | 0.041 | 0.026 | 0.041 | 0.011 | |||
| Std. Deviation | 0.0015 | 0.006 | 0.867 | 0.062 | 0.105 | 0.625 | 1.138 | 0.131 | 0.081 | 0.128 | 0.035 | |||
| Min.-Max. | 0.316-0.363 | 0.075-0.093 | 16.074-18.978 | 0.348-0.484 | 1.395-1.746 | 47.323-49.371 | 26.189-28.947 | 0.728-1.200 | 0.872-1.153 | 1.482-1.881 | 0.097-0.180 | 0.230-0.279 | ||
| ∑FFA/∑n | 0.341 | 0.082 | 18.038 | 0.422 | 1.497 | 48.537 | 27.831 | 1.003 | 0.98 | 1.691 | 0.146 | 20.661 | 79.565 | 0.26 |
| ∑FFA | 3.41 | 0.822 | 180.38 | 4.217 | 14.968 | 485.37 | 278.305 | 10.027 | 9.804 | 16.909 | 1.455 | 206.612 | 795.652 | 2.598 |
Table 2: Fatty acid profiling/ composition of variously treated bran oil extracted from parboiled rice samples. *Sign shows the essential fatty acids.
SAFFA = total saturated free fatty acids, USFFA = total unsaturated free fatty acids, FFA = total free fatty acids, FFA/n = average of total free fatty
acids, SLI = Shelf life index, C14:0 = Myristic acid, C14:1 = Myristoleic acid, C16:0 = Palmitic acid, C16:1 = Palmitoleic acid, C18:0 = Stearic acid,
C18:1 = Oleic acid, C18:2 = Linoleic acid, C18:3 = Linolenic acid, C20:0 = Arachidic acid, C20:1 = Eicosanoic acid, C22:0 = Behenic acid.
Myristic acid (C14:0)
It was found in traces (0.321) in raw and parboiled (0.341) rice bran oil samples (Tables 1 and 2). It ranged from (0.281-0.332%) and (0.316-0.363%) in raw and parboiled rice bran oils. Least traces were found in RT4 (0.281%) and highest percentage was observed in PT8 (0.363%).
Myristoleic acid (C14:1)
It was also observed in traces in raw (0.104) and parboiled (0.082) rice bran oils treated with different chemicals (Tables 1 and 2). It ranged from 0.089-0.115% in raw and 0.075-0.093% of parboiled rice bran oils and highest percentage was observed with the treatment RT10 (0.115%) among all the treatments on parboiled as well as unparboiled rice bran oil samples.
Palmitic acid (C16:0)
It was observed in parboiled rice bran oils (18.038) slightly higher contents compared to its contents in raw rice bran oils (16.538). It ranged from 8.872 to 18.691% in raw and 16.074 to 18.978% in parboiled rice bran oils in all the treatments. Treatment PT6 gave the highest content of palmitic acid in its oil (Tables 1 and 2).
Palmitoleic acid (C16:1)
It was found to show higher contents in rice bran oils of raw (0.563) on average basis, compared to that in oils of parboiled rice samples (0.422). It ranged from 0.5046 to 0.588% and from 0.348 to 0.484% in raw and parboiled rice samples respectively in all the treatments. RT7 showed highest contents of palmitoleic acid (0.588%) in its oil among all the oils treated with different chemicals (Tables 1 and 2).
Stearic acid (C18:0)
In case of stearic acid (C18:0) RT6 was found to have higher ratio (2.244%) among all the treatments. Its contents were found higher in raw rice on average basis (2.029) as compared to that in parboiled (1.497) and ranged from 1.914 to 2.244% in raw while from 1.395 to 1.746% in parboiled rice bran oil samples (Tables 1 and 2).
Oleic acid (C18:1)
Same as in case of palmitic acid, oleic acid (C18:1) was also found in highest ratio (49.371%) in PT6 among all the treatments. On averaged data basis, parboiled rice bran oil samples showed lower (43.386) as compared to 48.537 in oils of parboiled rice brans. The fatty acid contents ranged from 41.4051 to 44.175% and from 47.323 to 49.371% in raw and parboiled rice bran oil samples (Tables 1 and 2).
Linoleic acid (C18:2)
Linoleic acid (C18:2) was observed in higher quantity in raw rice samples (31.335) as compared to parboiled samples (27.831) on average basis. It ranged from 26.189 to 28.947% and 30.224 to 32.319% in raw and parboiled rice bran oils respectively. Liolenic acid (C20:0) ranged from 0.728 to 1.201 in parboiled and from 1.336 to 1.553% in raw rice bran oil samples. RT8 and RT7 were found to show highest proportion of linoleic (32.319%) and linolenic (1.553%) acids among all the treatments (Tables 1 and 2).
Eicosanoic acid, Eicosanoic acid and Behenic acid
Likewise, treatments PT1, PT7 RT7 showed highest contents of arachidic acid (1.153%), eicosanoic acid (1.882%) and behenic acid (0.613%) among all the treatments while treatments range of arachidic acid (0.872-1.153% and 0.660-6.005%) and eicosanoic acid (1.482-1.881% and 1.541-1.631%) for parboiled and raw rice bran oils respectively (Tables 1 and 2). On average basis, fatty acid contents of arachidic acid and eicosanoic acid was higher in parboiled (9.804 and 16.909 respectively) than in raw rice bran oil (12.063 and 16.091 respectively) as depicted in Tables 1 and 2. Behenic acid (C22:0) was much higher in raw rice (4.367) than parboiled (1.455) rice bran oils. It ranged from 0.215-0.612 in raw and 0.097-1.455 in parboiled rice bran oils for all the treatments. Treatment RT7 was also found to have highest contents of behenic acid (0.612%) among all the treatments.
Shelf life index (SLI)
The USFFA content in raw rice bran oils (784.69%) (Table 1 & 2), was about four times greater than the SAFFA (202.09%) in all treatments. Same results were observed in case of parboiled rice bran oil samples (206.61% and 795.65% for SAFFA and USFFA respectively). These results are in near agreement with Zhout et al. [13]; Oluremi et al. [7] and Tead et al. [19] and other researchers [14,16,17] as well dealing with fatty acids. Level of essential fatty acids indicates the shelf life of the oil. The higher the level of USFFA, the shorter the shelf life of oil [18-22]. By comparing lower value of total shelf life index (SLI) of raw rice bran oils collectively with that of parboiled rice bran oils indicating that parboiling increases the shelf life of oil by lowering proportions of unsaturated fatty acids and increasing saturated free fatty acids contents in oil. Treatment (RT4) of raw rice bran oil with 7% acetic acid gave highest SLI value (0.32) followed by 30 ml/kg HCL (RT4) giving SLI value of 0.28. The lowest value (0.15) of SLI was obtained for the treatment (RT6) of 30 mg/kg HCL with raw rice bran oil. Higher SLI values designate higher shelf life. Steaming parboiled rice at 100°C for 20 min gives second highest SLI value after sodium metabisulphite (1.0%) treatment (PT7) as indicated in Table 2.
Essential and non-essential fatty acids
The results further revealed that essential fatty acids (linoleic and linolenic acid) were found highest in raw rice bran oil treated with sodium metabisulphite (1.5%) and sodium metabisulphite (1.0%) respectively (Tables 1 and 2). Sodium Metabisulphite (1.0%) when treated with raw rice bran oils gave highest peaks of palmitoleic acid, linolenic acid and behenic acid while giving highest peaks of eicosanoic acid in parboiled rice bran oils. Likewise, 1.5% sodium metabisulphite when treated with raw rice bran oils gave highest peaks of linoleic acid whereas it gave highest peaks of myristic acid in parboiled rice bran oils. On the other hand, 30 ml/kg HCL and 1.0% H2SO4 treatment with parboiled rice bran oils gave highest peaks of palmitic acid and arachidic acid respectively. Steaming raw rice at 100°C for 20 minutes produced highest contents of myristoleic acid. It has been reported earlier that mammals such as human lack the ability to introduce double bonds in fatty acids beyond carbon 9 and 10, hence ω-6 linoleic acid (18:2,9,12), abbreviated LA (18:2n-6), and the ω-3 linolenic acid (18:3,9,12,15), abbreviated ALA (18:3n-3), are essential for humans in the diet.
Oleic, linoleic and palmitic acids were dominant fatty acids in all the parboiled and un-parboiled rice bran oil samples. The total content of fatty acid (FFA) of parboiled bran oil was found higher than that of unparboiled rice bran oil samples. While the other fatty acids were found less in bran oils of differently treated parboiled rice as compared unparboiled samples. From the obtained results, it may be concluded that treating raw rice bran oil with 1.0% and 1.5% Sodium Metabisulphite give more fatty acid contents as compared to other treatments. However, heating and extrusion methods may be used as better alternatives of using other harmful chemicals. It may further be concluded that one of the essential fatty acids i.e., linolenic acid (C18:3) is present in minute quantities on rice bran while the other essential fatty acid linoleic acid (C18:2) is in higher proportions as compared to other fatty acids. Rice bran oil therefore can be utilized as a good source of linoleic (C18:2) i.e., an essential fatty acid.