Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Review Article - (2018) Volume 8, Issue 2
Today’s consumers are more aware of benefits and health-related effects of food and food compounds, and would like to get benefit of health promoting effects from the food consumed. It is well known that food can be deteriorated by microbiological, chemical and physical factors that their health-promoting effect of food as well as their bioavailability are diminished or lost. Food are processed to extend the shelf-life with elimination of the detrimental effects, but most common food processing technologies cause denaturation of health-promoting compounds resulting in a decrease in food bioavailability. Thus, alternative technologies preserving nutritional and health-promoting effect of food and food compounds come forward. High pressure processing (HPP) as one of the leading nonthermal food processing technologies provides safety of food with fresh-like properties with minimal lost on nutritional and sensory properties; however, limited number of studies involve bioavailability of these compounds.
Keywords: High pressure processing; Bioavailability; Food component; Health-promoting effects
HPP: High Pressure Processing; PEF: Pulsed Electric Fields; IC50: Half Maximal Inhibitory Concentration; RS: Resistant Starch; TS: Total Starch; DS: Digestible Starch; AA: Amino Acids; BR: Brown Rice; 5MTHF: 5-Methyltetrahydrofolate.
Foods provide essential nutrients, growth factor, immune boosting compounds and other bioactive compounds (vitamin C, carotenoids, phenolic compounds, vitamin E, glucosinolates) with antioxidant, antitumoral and anticancerogenic properties for human body [1-3]. Today, the link between bioactive compounds taken by the diet and occurrence of some disease are well known. As lack of some nutrients causes some health problems such as scurvy, beri beri, etc., compounds formed by food processing technologies such as hydroxy methyl furfural (HMF), acrylamide, aldehyde and ketones can cause some metabolic disorders and even cancer. The diet is also associated with the morbidity and mortality in the chronic diseases, such as cardiovascular disease, cancer, hypertension and obesity. It is reported by several studies that diet is attributed to one-third of all cancer cases and one-half of cardiovascular diseases and hypertension [4,5]. Thus, it is important that consumed food and food compounds should provide essential nutrients for human body with minimization of health hazard effect. On the other hand, the metabolic pathway of each compound in addition to their bioavailability is important to determine their usage by human body. In order to be bioavailable, a food compound must be released from the food matrix and modified in the gastrointestinal (GI) tract. Moreover, the stability of the compound affecting its bioavailability and their possible beneficial effects is important before concluding on any potential health effect (Figure 1) [6].
Figure 1: Bioavailability definition [6].
One of the important terms to explain the use of a bioactive compound by human body, bioaccessibility, is defined as the fraction or the quantity of the compound released from the food matrix in GI tract which can be absorbed [7]. Food need to go through digestive transformations to be converted into material ready for assimilation, the absorption/assimilation into intestinal epithelium cells, and lastly, the pre systemic metabolism (both intestinal and hepatic) need to occur for bioaccesibility of a component. If the definition is solely on absorption based, then the beneficial effects of unabsorbed nutrients (such as binding of bile salts by calcium in the tract) would be missed. Therefore, generally simulating gastric and small intestinal digestion, sometimes followed by Caco2 cells uptake is the usual evaluation for in vitro digestion procedures for some nutrients [8]. Utilization of a specific nutrient is also important, and thus, the term bioavailability is also described as the utilization of a nutrient, and therefore, can be defined as the fraction of ingested nutrient or compound that reaches the systemic circulation and is utilized [9]. In general, bioavailability involves GI digestion, absorption, metabolism, tissue distribution, and bioactivity of a certain compound. Studies including bioavailability of a compound –therefore- must reveal that the component analyzed is efficiently digested and assimilated and then, once absorbed, exerts a positive effect in human health with considering bioactivity [10]. Bioavailability of a compound need to be determined in vivo in animals or humans as the area under the curve (plasma-concentration) of the compound obtained after administration of an acute or chronic dose of an isolated compound or a compound-containing food [11]; whereas bioactivity is the specific effect upon exposure to a substance including tissue uptake and the consequent physiological response (such as antioxidant, anti-inflammatory) that can be evaluated in vivo, ex vivo, and in vitro) [12].
Transformation of the fraction of food components by digestion into potentially accessible matter through all physical–chemical processes that take place in the lumen is defined as digestibility. Assimilation is another term that needs to be defined, and it refers to the uptake of bioaccessible material through the epithelium by some mechanism of transepithelial absorption [13].
Food processing technologies may alter, change or diminish the bioaccessibility of a specific compound by denaturation or causing changes in the structure. For most of the compounds, changes in the physical structure or denaturation may even direct toxic, mutagenic, carcinogenic and teratogenic effects. Thus, detailed studies need to be conducted to determine effect of particular processing technologies on bioaccessibility of food compounds. It is reported by numerous studies that consumers have a growing preference for convenient, fresh like, healthy, minimal-processed food products with natural flavor and taste and extended shelf-life. In order to meet these demands, alternative not-thermal preservation technologies as high pressure processing (HPP), pulsed electric fields (PEF) irradiation, light pulses, and natural bio-preservatives together with active packaging without compromising safety have been proposed [14,15]. HPP carried out with intense pressure in the range of 100-1000 MPa with or without heat is a promising “non-thermal” technique for food preservation allowing most foods to be preserved with minimal effect on taste, texture or nutritional characteristics. HPP was found superior to thermal sterilization and pasteurization due to the maintenance of sensory and nutritional characteristic of treated food products [16,17]. Both liquid and high-moisture-content solid foods are subjected to pressure treatment at relatively low temperature being lethal to microorganisms, but not effective to covalent bonds representing a unique characteristic of this technology because HPP has a minimal effect on food chemistry [17,18]. HPP retains food quality while avoiding the need for excessive thermal treatments or chemical preservation; however positive effect of HPP on bioavailability and bioaccessibility of food components need to be proven in order to claim that this technology provides preservation of functional properties. It is well known fact exposure of plant foods to HPP having changes at different magnitude on the nutritional properties and possible protective effects of the food once processed causes alteration on plant matrix structures. For example, HPP processing of green beans at 600 MPa provides a significant increase in lutein availability compared to untreated samples and this positive effect can be due to facilitated release of lutein within the plant tissue matrix during the in vitro digestion process by the disruption of cellular structures in the beans by exposure to high pressures. It is recommended by studies that possible changes on plant the tissue matrix such as disruption of plant cell walls induced by HPP results in the release of compounds with antioxidant actions into the extracellular environment [19,20].
Even though bioaccessibility and bioavailability of polyphenols, carotenoids, and glucosinolates may be enhanced by HHP as their extractability is increased by HPP [21,22], the extractability and bioavailability of nutrients and correlation between process-induced matrix disruption are not directly related to each other [22]. Bioassesibility of certain compounds, carotenoids for example, is more complex, in that, the positive effect of HPP would be anticipated based on its effect on food matrix structure [23]. Moreover, it is revealed that the impact of processing on bioaccessibility and bioavailability of nutrients, in general, is dependent on the type of nutrient, the structure and composition of the food matrix and the processing technique employed [22].
Although bioavailability or bioaccessibility of different food components were reported by both in vivo and in vitro studies, the effects of the food matrix on the bioavailability or bioaccessibility of antioxidant minerals and starch as well as effect of HPP have not been reported. Digestion and absorption of starch can be influenced by the direct interactions between this component and some components of food, such as binding to proteins and polysaccharides. Although HPP was proven to preserve nutritional properties; processing of apples at 500 MPa for 2, 4, 8 and 10 min significantly affect the antioxidant activity, mineral and starch content and bioaccessibility of apple samples. In vitro digestion has a noticeable effect on the antioxidant concentration, half maximal inhibitory concentration (IC50), with much lower values (a smaller IC50 value corresponds to a higher antioxidant activity) of apple samples compared with those untreated and nondigested. Calcium, iron and zinc bioaccessibility of apple samples in vitro was calculated as the percentage of the element dialyzed of the total amount present in the aliquot (% dialyzability) by the following equation:
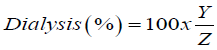
where Y is the element content of the dialysates mineral fraction (mg mineral element/100 g), and Z is the total mineral (calcium, iron or zinc) content of the sample (mg mineral element/100 g grain). Calcium, iron and zinc content of unprocessed samples are measured as 30.33 ± 1.94 mg/100 g, 14.46 ± 3.49 mg/100 g and 6.22 ± 0.91 mg/100, respectively and HPP provides an increase in the mineral content availability by 2.11-303.00% for calcium, 4.63-10.93% for iron and 8.68-28.93% for zinc. Moreover, both the dialysability and solubility of calcium, iron and zinc with respect to the values for the untreated sample are found to be reduced by HPP. HPP-treated samples exhibit higher antioxidant capacity with in vitro digestion and long time than that of the non-digestion samples suggesting an increase in the amount of antioxidants released by the apple matrix into the human intestine, and hence the antioxidant capacity of these samples, may be higher than expected from the data based on chemical extracts. Antioxidants are potentially available in the small gut; the degree to which they produce an antioxidant effect depends on the rate of absorption and this fact need to be considered when evaluating the antioxidant capacity of a fruit from a nutritional standpoint. Moreover, if the antioxidants are not released in the digestive enzymatic extracts, they may enter the colon where they can be fermented by the microflora, yielding different compounds that may be metabolized and may provide an antioxidant environment [24]. Consumption of apple under HPP may supply substantial antioxidants, mineral and starch, which may provide health promoting and disease preventing effects [24].
The sum of starch and the product of starch degradation not absorbed in the small intestine but is fermented in the large intestine of healthy individuals is described as resistant starch (RS) [24,25]. It has been released that RS participates in the reduction of glycemic and insulinemic responses to food and it has hypocholesterolemic effects and protects against colon rectal cancer [26,27]. Thus, the RS content in food, as well as the digestive rate and level of starch, has a positive effect on health [28]. It is indicated that increased treatment time at 500 MPa from 2 to 10 min causes significant decrease in RS content of the apple samples compare to untreated ones having 19.6 ± 1.9% of RS. Moreover, total starch (TS) of the untreated samples (81.2%) have increased to 99.8 % under 500 MPa for 10 min processing conditions. The TS is measured as = digestible starch (DS) + RS, and it ranges between 95.1% (untreated sample) and 103.5% (500 MPa for 10 min). Based on the HPP processing of apple, it can be said that consumption of HPP may supply substantial antioxidants, minerals and starch which may provide health promoting and disease preventing effects [24].
Effect of HPP at 0.1, 100, 300 and 500 MPa for 10 min on mineral elements, amino acids (AAs), antioxidants and starch on germinated brown rice (BR) at 37°C for 36 h has revealed that while the in vitro bioaccessibility of calcium and copper increases by 12.59-52.17% and 2.87-23.06% after HHP, respectively, bioaccessible iron is decreased [29]. Effect of HPP is obvious especially on AAs in that particularly indispensable AAs and gama-aminobutyric acid, as well as bioaccessible total antioxidant activities and starch resistance to enzymatic hydrolysis, significantly improved by HHP. Starch digestibility, on the other hand, increased by germination. HPP above 300 MPa causes structural changes in bran fraction revealing relationship between germination and HPP on nutrients bioaccessibility and develop appropriate processing conditions [29].
Changes on bioaccessibiliy of carrot, broccoli and green beans carotenoids is investigated after HPP processing of 400–600 MPa pressure and 2 min processing time. Among those, the bioaccessibility of carotenoids in carrot is not significantly changed. However, a slight improvement in lutein bioaccessibility in green beans and reduction on β-carotene bioaccessibility in broccoli is observed by HPP at 600 MPa [19].
Bioaccessibility of some compounds can be affected by physical state of the plant tissue. For example, the bioaccessibility of β-carotene in carrot tissue is inversely related to hardness [30]. Both mild thermal pasteurization and mild HPP pasteurization cause similar reduction in firmness, but tissue softening caused by these two processes have different physical effects on cell structure. Tissue softening provided by mild HPP in carrot tissue is mainly due to turgor pressure loss explaining the higher bioaccessibility of β-carotene in the sample compared to mild thermal pasteurization. On the other hand, thermal processing applied at more intense pasteurization and sterilization conditions results in a softer tissue compared to HPP resulting in higher bioaccessibility [31].
Studies related to bioavailability of high pressure processed orange juice vitamin C is somewhat controversy. Although no significant difference is observed between the fresh and HP processed orange juice vitamin C in one study [32], consumption of 500 mL per day of HP-treated orange juice at 400 MPa pressure, 40°C processing temperature for 1 min significantly elevates plasma vitamin C concentration in healthy subjects, indicating that vitamin C in HP-treated orange juice is bioavailable [33]. In addition, the level of biomarkers of oxidative stress and inflammation, F2-isoprostanes, uric acid, C-reactive protein and prostaglandin E2 also are lowered significantly at the end of the 14-day study, indicating that consumption of orange juice may help to decrease the risk of chronic diseases. HP- treated ‘gazpacho’ a traditional Spanish vegetable soup shows similar effect as the orange juice treated at 400 MPa pressure, 40°C processing temperature for 1 min [34].
Effect of HPP on antioxidant capacity, mineral and starch bioaccessibility of a nonconventional food: “algarrobo” Prosopis chilensis seed pressurized at 500 MPa during 2, 4, 8 and 10 min is measured through the antioxidant activity, mineral and starch content and bioaccessibility. All treatments provide an increase in the bioaccessibility of the antioxidant activity (IC50), minerals (dialysis and solubility) and starch (resistant and digestible) compared to the untreated sample of algarrobo samples. Bioaccessibility of calcium, iron and zinc in the treated sample for 500 MPa at 10 min, expressed as percentage solubility, is found several-fold higher (three, three and five times, respectively) than that of the untreated sample. Similar effect is also observed in IC50 value in that the untreated samples exhibit the lowest antioxidant activity (0.11 ± 0.005 mg/ mL) followed by all treated samples at 500 MPa for 2, 4, 8 and 10 min [35].
HPP may also influence the bioavailability of nutrients through its effect on the activity of endogenous enzymes such as folates in vegetable sources usually exist in the less bioavailable polyglutamate forms. The endogenous enzyme, γ-glutamyl hydrolase, hydrolyses the polyglutamyl folates into the more bioavailable short-chain or monglutamyl folates during cell decompartmentalization. Once this enzyme is inactivated by different processes such as freezing usually preceded by blanching, keeping the long chain polyglutamyl folates causes reduction of bioavailability of folates [36]. Unlike freezing usually preceded by blanching, cell decompartmentalization without inactivation of the enzyme and facilitation of the hydrolysis of long chain polyglutamyl folates potentially improving their bioavailability is enabled by HP treatment [22].
Effect of different processing technologies at both pilot and large scales and comparison of the pilot scale study with blanching (boiling water, 10 min); freezing (−18 and −80°C) followed by refrigerated (4°C) thawing and blanching; freeze-drying, followed by rehydration, 6 h refrigeration and blanching; and HP (50–200 MPa/RT/5 min), followed by 6 h storage and blanching on the formation of monoglutamate folates in leeks results in highest yield of monoglutamate folates when the samples are processed by HP (200 MPa/RT/5 min) and freezing (−18°C/16 h) and thawing (4°C, 24 h). Among the larger scale process of freezing–thawing–blanching and HP (200 MPa/RT/5 min), blanching causes the highest loss of total folates of 85% in leeks, 65 and 55% respectively in cauliflower and 79 and 81%, respectively in green beans. Blanched or steamed samples regardless of the subsequent process provide the lowest total losses of 16- 38% in the samples. Both blanching and steaming cause a reduction in the proportion of monoglutamate folates in all the vegetables compared to the raw sample from 33% to 6 and 11% respectively in leeks, from 9% to 3 and 2%, respectively in cauliflower and from 33% to 7 and 2%, respectively in green beans. The samples subjected to blanching prior to freezing or HP present similar reductions. However, samples subjected to HP or HP followed by blanching results in an increase in proportion of monoglutamate folates to 74 and 65%, respectively in leeks and 82 and 72%, respectively in green beans, whereas the effect is less significant in cauliflower with 9 and 12%, respectively after HP and HP–blanching. As a result, it is seen that freezing–thawing–blanching has a similar effect of significantly boosting the proportion of monoglutamate folates in all the vegetable [37].
HPP at 300, 450 and 600 MPa and 30°C for 0 and 5 min to quantify the influence of pressure on the polyglutamyl chain of 5-methyltetrahydrofolate (5MTHF) in carrot greens, baby carrots and cauliflower provides a significant increase in the conversion of 5MTHF to short chain and monoglutamyl forms and the highest extent of conversion to the monoglutamyl forms at 600 MPa and 5 min with 4, 23, and 2.5 fold increases of the monoglutamyl content in cauliflower, baby carrot, and carrot greens, respectively [38].
Bioaccesibiliy is one of the important term to explain the usage of a certain food components by human body. Although bioaccessibility of different compounds are measured by both in vivo and in vitro studies, exact measurement is affected by different factors such as method of measurement, analysis conditions, and physical state of the plant tissue in which the compounds will be extracted from, the pretreatment of the samples, etc. Studies reveal that different results can be obtained even between in vivo and in vitro experiments for the bioaccessibility of the same compounds. Food processing altering or causing changes in the plant cells structure and HPP causing structural changes in plant cell affect the bioaccessibility of the compounds. On the other hand, studies involving measurement of HPP influence on bioaccessibility have different results as HPP depending on the magnitude of pressure, processing time and processing temperature present a great variability for bioaccessibility of same and different compounds. Thus studies involving the bioaccessibility of same compound under different HPP processing conditions or different compounds under the same HPP processing conditions may end up with different results. But, based on the previous reports, one can be sure that HPP in general have a positive effect on bioaccessibility. However, more studies need to be conducted to explore the effect of HPP on bioaccessibility of different compounds under different processing conditions.