Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2020)Volume 10, Issue 4
Background: Low-carbohydrate diets have been a popular nutrition therapy since the American Diabetes Association’s 2013 recommendation. Low-carbohydrate diets are effective at lowering blood glucose and hemoglobin A1c levels. Here, we suggested the new diets intake restriction methods using low-sugar, high-dietary fiber noodles for those consuming noodles regularly. This study aimed to investigate the effect of a 28 day low-sugar, high-dietary fiber noodles diet, containing okara and konjac, on blood glucose levels of Spontaneously Diabetic Torii fatty rats, a model for obese type 2 diabetes.
Methods: Male, 7-week-old rats were divided into two groups. Group I was fed the AIN-93G as standard diet (n=6) as control group, and group II was fed the standard diet in which 50% dried low-sugar, high-dietary fiber noodles replaced cornstarch (n=6). Body weight, food intake, and blood glucose levels were measured once a week, and hemoglobin A1c and glycated albumin levels analyzed after 28 days administration. Following the 28 days, the rats were fasted, glucose (2,000 mg/kg body weight) was administrated for the oral glucose tolerance test.
Results: The blood glucose, hemoglobin A1c, and glycated albumin levels of the low-sugar, high-dietary fiber noodles group were significantly lower than those of the control group, in which body weight gain was observed.
Conclusion: This is the first report on the effectiveness of low-sugar, high-dietary fiber noodles on blood glucose levels in model rats. The low-sugar, high-dietary fiber noodles might be beneficial for diabetes or celiac disease patients who consume noodles.
Low-sugar high-dietary fiber noodles diet; Blood glucose; Body weight
Type 2 diabetes mellitus (DM) is a chronic metabolic disorder. The morbidity of diabetes is expected to increase from 415 million in 2015 to 642 million by 2040 [1]. The American Diabetes Association and the British Diabetic Association reported that low-carbohydrate diets (LCD) may be effectively used as a medical nutrition therapy to manage blood glucose and lipid levels, and LCD is effective at lowering blood glucose and hemoglobin A1c (HbA1c) levels [2-4].
Grains such as rice and noodles are the primary carbohydrate source for almost all the Asian populations [5]. Zuñiga et al. reported that high consumption of noodles was associated with a higher risk of type 2 diabetes alike the case of high rice consumption [6]. Therefore, we have developed low-sugar, high-dietary fiber (LSHDF) noodles containing a by-product of soybean (Glycine max) processing (commonly called “okara”) and Amorphophallus konjac (commonly called “konjac”) for those who consume noodles regularly. So far, there are no experimental data on the effect of LS-HDF noodles intake on blood glucose levels over a 28 days administration.
Many people have been interested in reactive oxygen and antioxidants. Because oxidative stress has been linked to the generation of various serious diseases like neurodegenerative disorders, cancer, cardiovascular diseases, atherosclerosis, cataracts, and inflammation [7]. Moreover, there are reports that insulin resistance in type 2 DM could be caused by oxidative stress, inflammation, glucotoxicity and lipotoxicity [8,9].
In this preliminary study, the antioxidant activity of LS-HDF noodles were measured as general information, and we demonstrated the effect of LS-HDF noodles on blood glucose levels in Spontaneously Diabetic Torii fatty rats (SDT-fr), a model for obese type 2 diabetes for the first time.
Materials
The commercial LS-HDF noodles (Healthy Noodles™ from Kibun Foods Inc. in Japan) were lyophilized. In brief, the okara were mixed with konjac and other ingredients and then boiled. Raw LS-HDF noodles contains approximately 1.3% protein, 0.8% fat, 0% sugar, 6.5% dietary fiber and 19.4 kcal/100 g energy. Folin & Ciocalteu’s phenol reagent (2N) was purchased from Sigma- Aldrich Co. (St. Louis, MO, USA). 2-Morpholinoethanesulfonic acid monohydrate (MES) was obtained from Dojindo Laboratories (Kumamoto, Japan).
Extraction of LS-HDF noodles for measurement of antioxidant activity
Extraction of LS-HDF noodles with organic solvent was performed using methods of our previous report [10,11], with some modification. In brief, the powder of lyophilized LS-HDF noodles was extracted with 10 times the volume of 50% acetone at 23-27°C, and the extracted solution was concentrated. The solution was used for later experiments as LS-HDF noodles extract solution.
Total phenolic content
The total phenolic content of LS-HDF noodles was analysed by the Folin & Ciocalteu method with catechin as a standard [12] and our previous report [11]. The LS-HDF noodles extract solution (20 μL) was mixed with 20 μL of 1N Folin & Ciocalteu solution and 100 μL of 0.4 M sodium carbonate solution. The reaction mixture was stored at 30°C for 30 min, and the absorbance was measured using a Synergy HTX Multi-Mode Microplate Reader (BioTek Instruments, Winooski, VT, USA) at 660 nm. Results were expressed as milligrams of catech in equivalent per gram of dry weight of LS-HDF noodles (mg catechin/g dry powder). 1,1-diphenyl-2-picryl-hydrazyl (DPPH) free radical scavenging activity
The DPPH radical scavenging activity of LS-HDF noodles was analysed by the methods described by Midoh et al. [13] and our previous report [11]. The LS-HDF noodles extract solution (60 μL) was mixed with 120 μL of 100 mM MES buffer (pH 6.0)/10% ethanol solution and 60 μL of 400 μM DPPH in ethanol. The reaction was performed at room temperature for 20 min, and the absorbance of the reaction mixture was measured at 520 nm using a microplate reader. The DPPH radical scavenging activity of fermented soybean was estimated using a 6-hydroxy-2,5,7,8- tetramethylchromane-2-carboxylic acid (Trolox) standard curve (0, 5, 10, 15, 20, and 25 μM) and expressed as μmol Trolox/g LS-HDF noodles dry powder.
Oxygen radical absorbance capacity (ORAC) assay
The ORAC was performed using the OxiSelectTM ORAC Activity Assay Kit (Cell Biolabs Inc., San Diego, CA, USA) [14] and our previous report [11]. The reaction was performed in 75 mM phosphate buffer (pH 7.4), and the volume of the final reaction mixture was 200 μL. Antioxidant (25 μL) and fluorescein solution (150 μL) were added to the microplate wells. The reaction mixture was incubated at 37°C for 30 min. The free radical initiaor 2,2'-azpbis (2-methylpropionaidine) dihydrochloride (25 μL) was added using a multichannel pipette. The microplate was immediately placed in the reader, and the fluorescence intensity (excitation at 485 nm, emission at 528 nm) was recorded every 5 min for 60 min using a microplate reader. The area under curve at specific Trolox concentrations (0, 5, 10, 20, 40, and 50 mM) was used to plot the standard curve for ORAC activity. Each extract was quantified and expressed as μmol Trolox equivalents/g of dry LS-HDF noodles powder.
Animal experiments
Male, 5-week-old SDT-fr, purchased from CLEA Japan, Inc. (Tokyo, Japan), were individually housed under a 12-h light/dark cycle at 23 ± 3°C. After 2 weeks of acclimatization, these rats were divided into two groups. Group I (without LS-HDF noodles) was fed the AIN-93G as standard diet (n=6), and group II (with LS-HDF noodles) was fed the standard diet in which 50% dried LS-HDF noodles replaced cornstarch (n=6). The animals were allowed free access to food and tap water ad libitum for 28 days. Body weight, food intake, and blood glucose levels were measured once a week, and HbA1c and glycated albumin (GA) levels were analyzed using an enzyme-linked immunosorbent assay after 28 days. Following a 28 day administration period, the rats were fasted, and 2,000 mg of glucose/kg body weight was administered for oral glucose tolerance test (OGTT). Animal experiments were performed at Japan Food Research Laboratories and authorized by the Japanese Government. The study was conducted according to the ethical guidelines for laboratory animals and the standard operating procedures of the laboratory. The experimental protocol was approved by the animal experiment ethics committee of the laboratory (approval no. TM18032001).
Diets
Rats in group I were fed the AIN-93G (Oriental Yeast Co., Ltd, Tokyo, Japan) as control diet. Rats in group II were given a diet in which dried LS-HDF noodles replaced cornstarch. Formulation and nutrients of experimental diets in this study are shown in Table 1.
Table 1: Composition of the experimental diets.
| Variables | Group I | Group II |
|---|---|---|
| (Without LS-HDF noodles) | (With LS-HDF noodles) | |
| Ingredients (wt%) | ||
| Casein | 20 | 20 |
| L-Cystine | 0.3 | 0.3 |
| Cornstarch | 52.9486 | 2.9486 |
| Sucrose | 10 | 10 |
| Soybean oil | 7 | 7 |
| Cellulose | 5 | 5 |
| Mineral mix, AIN-93G-MX | 3.5 | 3.5 |
| Vitamin mix, AIN-93-VX | 1 | 1 |
| Choline bitartrate | 0.25 | 0.25 |
| Butylhydroquinone | 0.0014 | 0.0014 |
| Dried LS-HDF noodles | - | 50 |
| Total (wt%) | 100 | 100 |
| Nutrition facts (/100 g) | ||
| Energy (kcal) | 400 | 309 |
| Protein (g) | 20 | 27.5 |
| Fat (g) | 7 | 11.4 |
| Carbohydrate (g) | 67.9 | 54.4 |
| Sugar (g) | 62.9 | 12.9 |
| Dietary fiber (g) | 5 | 41.5 |
Statistical analysis
Results are expressed as the mean ± standard error (SE) in vitro study and animal study. Statistical significance was determined using a paired-t test. A p-value of less than 0.05 was considered statistically significant.
Total phenolic content and antioxidant activity of LS-HDF noodles
Table 2 summarizes the results of the total phenolic content and antioxidant activity of LS-HDF noodles. The total phenolic content (mg/g dry powder) of LS-HDF noodles was 0.144 ± 0.002. DPPH radical scavenging activity (μmol Trolox/g dry powder) of LS-HDF noodles was 0.416 ± 0.003. The ORAC values (μmol Trolox/g dry powder) of LS-HDF noodles was 2.209 ± 0.098.
Table 2: Total phenolic content and antioxidant activity of LS-HDF noodles.
| Variables | LS-HDF noodles |
|---|---|
| Total phenolic content | 0.144 ± 0.002 |
| (mg/g dry powder) | |
| DPPH radical scavenging activity | 0.416 ± 0.003 |
| (µmol Trolox/g dry powder) | |
| ORAC | 2.209 ± 0.098 |
| (µmol Trolox/g dry powder) |
Results are expressed as mean ± SE (n=3).
Effect of LS-HDF noodles on blood glucose levels in SDT-fr Results in SDT-fr study are shown in Figure 1. No significant differences were seen in food intake between group I and group II (Figure 1A), and no deaths or abnormalities were observed in group II. The calories of food intake of group II were significantly lower (p< 0.01) than those of group I (Figure 1B). The blood glucose levels of group I increased with time and showed a marked rise on day 14. However, the blood glucose levels of group II remained stable for 28 days (Figure 1C). Body weight of both groups increased with time, but these levels significantly differed between groups I and II from day 21 (Figure 1D). The HbA1c level of group II tended to be lower than that of group I after 28 days (Figure 1E). The GA levels of group II were significantly lower (p< 0.01) than those of group I (Figure 1F).
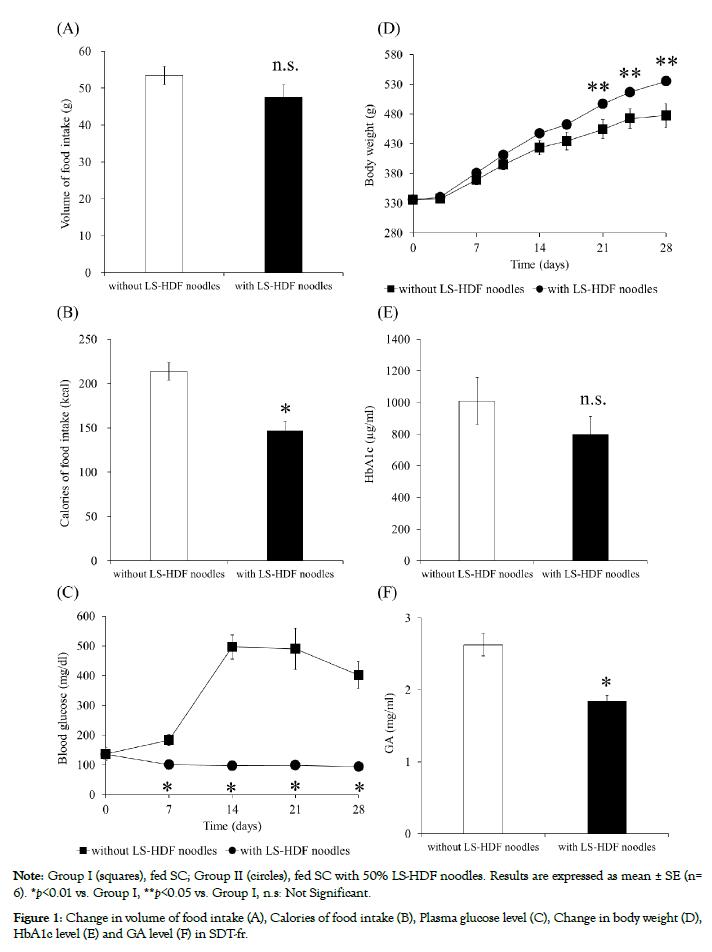
Figure 1: Change in volume of food intake (A), Calories of food intake (B), Plasma glucose level (C), Change in body weight (D), HbA1c level (E) and GA level (F) in SDT-fr.
After LS-HDF noodles administration for 28 days, OGTT indicated that the levels of plasma insulin and incretin, glucagonlike peptide-1 (GLP-1) and gastric inhibitory polypeptide (GIP) of group II were no different from those of group I (Figures 2A, 2B and 2C). In contract, the blood glucose levels of group II were significantly lower than that of group I at 60, 90 and 120 min after oral glucose loading (Figure 2D), and group II had an approximately 50% reduction in area under the curve (AUC) value compared to group I (Figure 2E).
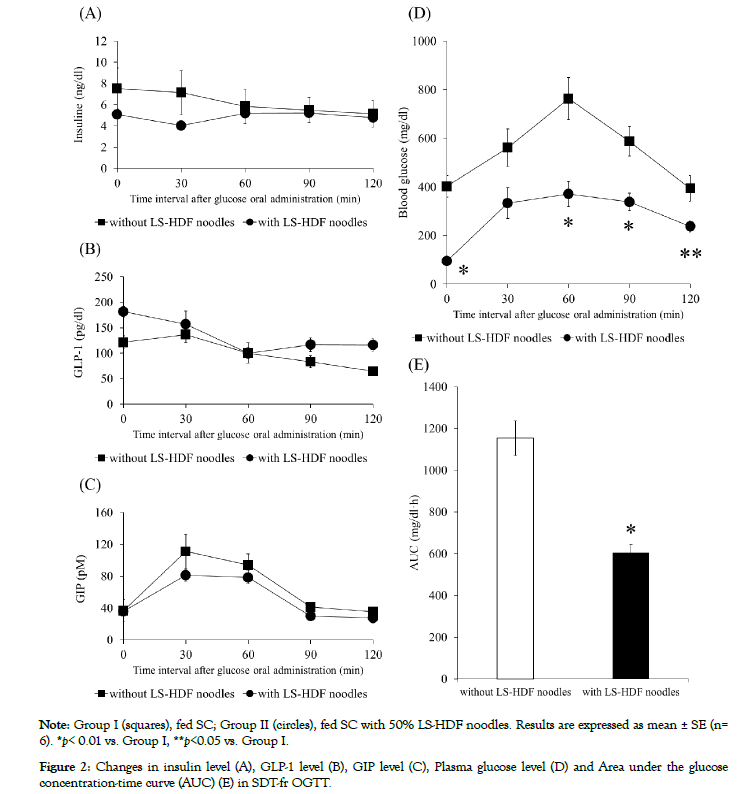
Figure 2: Changes in insulin level (A), GLP-1 level (B), GIP level (C), Plasma glucose level (D) and Area under the glucose concentration-time curve (AUC) (E) in SDT-fr OGTT.
The prevalence of type 2 diabetes has been gone up all over the world [15], and refined carbohydrates including rice and noodle have been suggested to deteriorate glucose metabolism [16]. However, rice and nood leare major staple foods of Asian people. Thus, we have developed LS-HDF noodles containing okara and konjac for those who consume noodles regularly. In this study, we carried out the effect of LS-HDF noodles on blood glucose levels in SDT-fr, a model for obese type 2 diabetes as preliminary research.
SDT-fr in group II were given a diet in which dried LS-HDF noodles replaced 50% of cornstarch. Therefore, the sugar levels of groups I and II were 62.9% and 12.9%, dietary fiber levels of groups I and II were 5.0% and 41.5% and the energy intakes of groups I and II were 400 kcal/100 g and 309 kcal/100 g, respectively (Table 1). Blood glucose levels of group II were significantly lower than those of group I during 28 days administration. On the other hand, interestingly, body weight of group II were significantly getting higher than those of group I from 21 days administration, despite blood sugar level and energy intake of group II being lower than those of group I. Now the mechanism of relationship between body weight gain and LS-HDF noodles intake is unclear, and we are assuming that reason of body weight gain are increasing of fat mass and/or lean body mass and effect of energy expenditure and so on. Volek reported that normal-weight men following an LCD for 6 weeks experienced a significant reduction in fat mass and an increase in lean body mass [17]. Manninen found that LCD causes body composition changes that favor loss of fat mass and preservation of muscle mass [18]. Moreover, Harber described that a low-carbohydrate/high-protein diet enhanced the rate of skeletal muscle protein synthesis [19]. Based on these reports, combined with our results, we assumed that the body weight increase in group II was due to the lean body mass increase as one reason of body weight gain, despite using LS-HDF diets not LCD. Additionally, the protein levels of groups I and II were 20.0 g/100 g and 27.5 g/100 g, respectively, supporting the assumption that the change in muscle mass of SDT-fr was caused by the intake of LS-HDF noodles. We intend to evaluate the relationship between body weight and muscle mass after the intake of LS-HDF noodles using our animal model.
The HbA1c level of group II tended to be lower than that of group I, and GA levels of group II were significantly lower than those of group I after 28 days administration. A deterioration of glycemic control is the primary concern in diabetes treatment and plays a key role in developing related complications [20]. The HbA1c level is used for long-term (2-3 months) glucose monitoring [21,22], and GA level for short-term glycemia monitoring, due to the ~3-week half-life of albumin [23]. In this study, the administration period of LS-HDF noodles to SDT-fr was short, supporting the value of GA measurement to assess glycaemic control in this study.
In OGTT after LS-HDF noodles administration for 28 days, the blood glucose levels of group II were significantly lower than that of group I. This finding suggested that intake of LS-HDF noodles for 28 days is effective on not only fasting blood glucose levels control but also suppression of blood glucose levels increasing after oral glucose administration. On the other hand, no marked differences were seen in the levels of plasma insulin, GLP-1 and GIP between group I and group II in OGTT. Kondo-Ando et al. investigated the effect of low-carbohydrate staple food (bread) on glucose metabolism in type 2 DM patients, and reported that postprandial blood glucose, insulin and GIP levels of low-carbohydrate staple food groups were significantly lower than control groups, but GLP- 1 levels did not differ [24]. There is a paper about the effects of lowcarbohydrate and enriched whey protein diet on anthropometric, hematochemical and cardiovascular parameters in subject with obesity, and the low-carbohydrate and enriched protein diets induced a significant decrease of insulin levels [25]. Higashida et al. found that low-carbohydrate high-protein diet suppressed the insulin response to glucose load in normal mice [26]. In this study, SDT-fr were used as model for type 2 diabetes. The rats showed hyperlipidemia from 4 to 8 weeks of age, blood glucose levels were increased with increasing age, on the other hand, the insulin levels of the rats decreased gradually [27]. Therefore, it is important to suppress the blood glucose levels increasing for preventing type 2 diabetes deterioration in this situation. Our results showed that LS-HDF noodles administration for 28 days was effective the suppression of blood glucose levels, and the insulin levels in group I and II were low in OGTT.
The LS-HDF noodles presented the antioxidant activities (DPPH radical scavenging activity and ORAC value), and phenolic compounds were contained in LS-HDF noodles. In our previous studies, we reported that soybean residue “okara” and its fermented form showed the reactive oxygen scavenging activity [28,29], and Vital et al. investigated the ferric reducing activity and DPPH radical scavenging activity of okara [30]. Moreover, there are some reports about antioxidant and anti-inflammatory effects of konjac glucomannan [31,32]. Insulin resistance in type 2 DM could be caused by oxidative stress, inflammation, glucotoxicity and lipotoxicity [8,9]. So, the LS-HDF noodles intake which had the antioxidant activity might be effective for preventing DM deterioration.
There are benefits and risks of an LCD in type 2 diabetes patients; LCD increases mineral deficiency and hypovitaminosis, and reduces dietary fiber intake [33,34]. In this study, the dietary fiber levels was 5.0% and 41.5% in groups I and II, respectively. This indicates that LS-HDF noodles can replenish dietary fiber in LCD. To the best of our knowledge, this is the first study to report the effect of LSHDF noodles on blood glucose levels in SDT-fr. However, in this preliminary study, we did not assess the effect of LS-HDF noodles on insulin sensitivity, glucagon levels, lipid profiles (triglycerides, total cholesterol, low-density, and high-density lipoprotein levels), relationship between body weight and muscle mass gain, effects of a long-term administration of LS-HDF noodles, and how LSHDF noodles will affect clinical study participants. Moreover, the mechanisms underlying blood glucose level suppression by LSHDF noodles remain unclear, and additional studies of its effect on blood glucose control are needed. Globally, many people and/ or patients with type 2 diabetes consume seasoned staple foods containing rice, bread, and noodles that are rich in carbohydrates; consequently it may be difficult to follow an LCD in daily life for long periods [35].
There are numerous methods for the management of type 2 diabetes, and one of the effective methods is dietary carbohydrate restriction such as an LCD. However, an LCD has both benefits and risks, and it is important to individualize the diet used. In this study, we described the effect of LS-HDF noodles in model animals, and we suggest new information about simple methods for the management of diabetes.
We are grateful to Chairman and CEO Masahito Hoashi and Rie Nozaki, Hideto Matsuzaka, Koji Hasegawa, and Masahiko Terayama, Kibun Foods Inc., for supporting this study.
This work was performed without an external funding source.
The author(s) have no conflict of interest to report.
Citation: Suruga K, Tomita T, Kadokura K (2020) Effect of Low-Sugar and High-Dietary Fiber Noodles Diet on Blood Glucose Levels in Spontaneously Diabetic Torii Fatty Rats. J Nutr Food Sci. 10:777. doi: 10.35248/2155-9600.20.10.777.
Received: 07-Jul-2020 Accepted: 20-Jul-2020 Published: 27-Jul-2020 , DOI: 10.35248/2155-9600.20.10.1000777
Copyright: © 2020 Suruga K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.