Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2014) Volume 4, Issue 6
Portulaca oleracea is globally used both as a vegetable and as an herb for medical and therapeutic purposes; hence the need to investigate its phytonutrients. The aim of this study was to evaluate the effects of different drying methods (hot-air drying, microwave drying and freeze-drying) on the color, phytochemicals content and antioxidant capacity of purslane leaves. The fresh purslane leaves had high contents of total phenolics (1447.59 mg GAE/ 100 g) and flavonoids (5011.87 mg QE/ 100 g) on dry weight basis. They exhibited high antioxidant capacity (53.23% and 147.78 μmol trolox/ 100 g) measured by DPPH and ABTS assay, respectively. Chromatic coordinates (L*, a* and b*) as well as total color difference (ΔE) were affected by drying methods. Drying methods caused a significant decrease in total phenolics, total flavonoids and antioxidant capacity of purslane leaves. Drying by hot-air at 50ºC and freezedrying had the lowest adverse effects on antioxidant capacities of purslane leaves while microwave drying cannot be a competitive process for preserving antioxidants and antioxidant capacity of purslane leaves. The changes in the antioxidant capacity due to the drying methods were positively correlated with the content of phenolics (R2= 0.9043- 0.9885). Therefore, it can be suggested that special care should be taken when processing method is selected for the exploration of purslane leaves.
Keywords: Purslane; Drying; Antioxidant activity; Phenolics; Flavonoids; Chlorophylls
Purslane (Portulaca oleracea L., Portulacaceae family) is a wellknown edible plant, widespread in temperate and tropical regions of the world. It is an herbaceous and annually plant with a fleshy stem and thick, green, succulent leaves and small black seeds that have medicinal properties. Purslane is listed by the World Health Organization as one of the most used medicinal plants and it has been given the term «Global Panacea» [1]. It has been described as a «Power Food» of the future because of its high nutritive and high antioxidant properties [2]. From the point of view of traditional medicine, the nature of purslane is cold and wet, astringent and diuretic, bile anodyne that relieves temperature of blood, liver and stomach. It is useful in the elimination of headaches, thirst relief, stoppage of bleeding, crushing of bladder stones and reduction of coughing and irritation of urethra, bladder, intestines, and hemorrhoids and used as a health food for patients with cardiovascular diseases [3]. The methanolic extract of purslane was found to exhibit moderate antimicrobial activity against Bacillus subtilis, which referred to a monoterpene glucoside named portuloside A [4].
Purslane contains vitamins B1, C and A, noradrenaline, dopamine, organic acids, coumarins, flavonoids and phenolic alkaloids [5,6]. It is a rich source of omega-3 fatty acids [7] and β-carotene [3]. It contains 17.50-29.04% protein, 5.00-12.00% crude fibers, 17.80-23.01% ash, 380-2625 mg/ 100 g calcium and 46-550 mg/ 100 g iron, on dry weight basis [8,9]. Lim and Quah [10] found that the total phenol content of six cultivars of purslane ranged from 127 to 478 mg/ 100 g of fresh weight of plant, and the methanolic extracts of all cultivars were capable of inhibiting lipid peroxidation. The aqueous extract of purslane does not have any cytotoxicity or genotoxicity effects and it is safe for daily use as a vegetable [11]. Purslane is one of the vegetable crops that are eaten extensively in soups and salads in Greece, Turkey and other Mediterranean countries. The tender stems and leaves can be eaten raw, cooked or pickled. The leaves can be frozen or dried and stored for many years [12].
Drying is a very common preservation method used in foodstuffs and the quality of the final products is strongly dependent on the technique and the process variables used [13]. The reduction of water activity by moisture removal leads to significant reduction of weight and volume, minimizing packaging, transportation and storage costs [14]. Drying also, alters other physical, biological and chemical properties of foods [12]. Hot-air drying is one of the most frequently used operations for food dehydration [15]. A major disadvantage associated with hotair drying is that it takes long time even at high temperature, which may cause serious damage to the flavor, color and nutrients in dried products [16-18].
Microwave drying is an alternative method to the conventional drying. It is rapid, more uniform, energy efficient, space utilization, prevents food decomposition and appears to have a high potential for the agricultural products processing [19]. The great interest in this technology is due to the high capacity of penetration of these waves, that heat not only on the surface but also inside the food. This speed up the drying process and can improve the quality of the final product [20].
Freeze-drying would be the best method of water removal with high quality final product compared to other drying methods. Some studies reported that freeze-drying increases the extraction of bioactive compounds of different products in comparison to air drying [21-23]. This is because it is based on the dehydration by sublimation of a frozen product [17]. Freeze-drying has been recognized as the most expensive process for manufacturing a dehydrated products and its application depends on the uses of the final product. So, till now freeze-drying technology is only used at an industrial scale to dry coffee, spices, meats and other high-value foods [24].
The studies on the drying characteristics of purslane leaves are scarce, and there are not enough studies on the effects of drying methods and their conditions on the properties of purslane. This study represents the first systematic analysis of the effects of three different drying methods (freeze, hot-air and microwave drying) on the antioxidant capacity, color attributes, phenolics and pigments of purslane leaves.
Plant material
Fresh Purslane (Portulaca oleracea L.) plants were harvested from a private fields (Ismailia and El-Sharqia Governorates, Egypt) prior to flowering period during August 2012. Green leaves were manually separated from plant, washed with water and then drained and left to dry on a cheese cloth for 15 min at room temperature (35 ± 2°C). The moisture content of the fresh leaves was immediately determined according to the AOAC [25] method (number 934.01), and found to be 90.78 ± 0.03 g water per 100 g sample.
Chemicals and reagents
Folin-Ciocalteu’s phenol reagent, anhydrous sodium carbonate, gallic acid, aluminum chloride and sodium hydroxide were purchased from Fluka. Sodium nitrite, quercetin, 2.2-diphenyl-1- picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman- 2-carboxylic acid (trolox), potassium persulfate and 2,2´-azino-bis (3-ethylbenzothiazoline–6-sulfonic acid) diammonium salt (ABTS) were obtained from Sigma-Aldrich CO. Methanol, hexane and acetone (analytical grade) were from Scharlab.
Drying Methods
Hot-air oven drying: One hundred grams of purslane leaves were distributed uniformly as a thin layer onto stainless steel trays of size 36.5 cm × 60 cm and dried in a convective dryer (WT-binder, type F115, Germany) at 50, 60 and 70°C at a constant air velocity (0.6 m/s) and ambient relative humidity. The drying time required to reach the equilibrium moisture content was 420 ± 30, 225 ± 37 and 105 ± 15 min and the moisture content of the dried leaves was 6.63 ± 0.33, 7.30 ± 0.08 and 7.18 ± 0.53 g water per 100 g leaves dried at 50, 60 and 70°C, respectively.
Microwave oven drying: A domestic microwave oven (Microchef 2335, Type 907, Moulinex, France) with maximum output of 1250W at 2450 MHz was used for the drying experiments. The dimensions of the microwave cavity were 335 mm × 330 mm × 195 mm. The microwave oven consisted of a rotating glass plate with 280 mm diameter at the base of the oven. Time adjustment is done with the aid of a timer located on the oven. A 100 g of purslane leaves were spread on the glass plate inside the microwave cavity and processed until the leaves were completely dried at three microwave output powers (360, 900 and 1250 W). The drying time required to reach the equilibrium moisture content was 54.0 ± 2.0, 25.0 ± 1.0 and 12.0 ± 2.0 min and the moisture content of the dried leaves was 7.90 ± 0.01, 4.30 ± 0.17 and 4.30 ± 0.23 g water per 100 g leaves dried at 360, 900 and 1250 W, respectively.
Freeze-drying (FD): A vertical freeze-drier (CPERON, FDU-7006, Gyeonggi, Korea) was used to freeze-dry the samples. One hundred grams of purslane leaves were placed in polyethylene page and treated at –20°C for 24 hours. The samples were then placed in the tubes of the freeze drier for 36 hours at -70°C. The moisture content of the dried leaves was 4.35 ± 0.33 g water per 100 g sample.
The drying experiments were conducted in triplicate. All samples were ground and passed through 250 μm sieve. The ground samples were stored at 4°C for further analysis.
Surface color measurement
The Color values of the samples were measured by a Minolta color reader CR-10 (Osaka, Japan). The measurements were expressed in L*, a*, and b* values which represents light–dark spectrum with a range from 0 (black) to 100 (white), the green–red spectrum with a range from -60 (green) to +60 (red), and the blue–yellow spectrum with a range from -60 (blue) to +60 (yellow) dimensions, respectively. Ten replicates measurements were performed and results were averaged. The total color difference (ΔE) was calculated by using the following equation according to [26] where L0, a0 and b0 are the control values for the fresh purslane leaves:
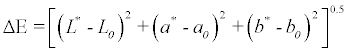
Determination of pigments
β-carotene, chlorophyll a and b contents were determined with the method described by Barros et al. [4] with some modifications as follows: A 500 mg of minced fresh or 200 mg of dried leaves powder was vigorously shaken with 10 ml of acetone-hexane mixture (4:6) for 5 min and filtered through filter paper No. 102. The extract was adjusted to 10 ml with volumetric flask. The absorbance of the extract was measured at 453, 505, 645 and 663 nm using a spectrophotometer (6505 UV/ VIS, Jenway LTD, Felsted, Dunmow, UK). Contents of β-carotene, chlorophyll a and b were calculated according to the following equations:
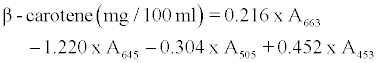
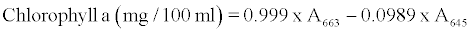
and further expressed as mg per 100 g dry weight.
Preparation of purslane leaves extract
The extract for determination the contents of total phenolics and flavonoids and antioxidant capacity of dried purslane leaves was prepared according to the method described by Barros et al. [27] with some modifications as follows: one gram of minced fresh leaves or fine dried powder was stirred with 25 ml of methanol at 100 rpm on Orbital Shaker (LAB-LINE Instruments, Inc., USA) for 1 h at room temperature (35 ± 2°C) and filtered through filter paper No. 102. The residue was then re-extracted with 25 ml of methanol. The methanol extracts were combined and stored at 4°C till further analyses. The extract was diluted if necessary.
Determination of total phenolics content
Total phenolics content was evaluated in the methanolic extracts, according to the Folin-Ciocalteu method with slight modifications [27]. One ml aliquot of the extract was mixed with 5 ml of Folin–Ciocalteu phenol reagent (diluted with water 1:10 v/v) and 4 ml of sodium carbonate (75 g/ L). The tubes were vortexed for 30 s and allowed to stand for 60 min at room temperature (35 ± 2°C) for color development. The absorbance was measured at 765 nm by spectrophotometer. A calibration curve (R2= 9995) of gallic acid (0-0.10 mg/ ml) was prepared and tested under similar conditions. The results were expressed as mg of gallic acid equivalents per 100 g of dry weight (mg GAE/ 100 g DW). All samples were analyzed trice and the results averaged.
Determination of total flavonoids content
Total flavonoids content was determined by technique reported by Barros et al. [27]. Shortly, 0.5 ml aliquot of the extract was mixed with 2 ml of distilled water followed by addition of 0.15 ml of NaNO2 (5%) solution. After 6 min, 0.15 ml of AlCl3 solution (10%) was added and allowed to stand for another 6 min before 2 ml of NaOH solution (4%) was added. The mixture was brought to 5 ml with distilled water. Then the mixture was mixed well and allowed to stand for 15 min. The absorbance was measured at 510 nm. A calibration curve of quercetin was prepared and total flavonoids content was determined from the linear regression equation (R2= 0.9999) of the calibration curve. The results were expressed as mg quercetin equivalents per 100 g of dry sample.
Determination of DPPH radical–scavenging activity
The antioxidant activity of the extract was measured by DPPH assay described by Ravichandran et al. [28] as follows: 0.1 ml of the methanol extracts was mixed for 30 s with 3.9 ml of DPPH solution (6 x 10-5 M). The solution was incubated at room temperature for 30 min, and the decrease in absorbance at 515 nm was measured at the end of incubation period with a spectrophotometer. The DPPH solution without extract was analyzed as control. The antioxidant activity was calculated as follows:
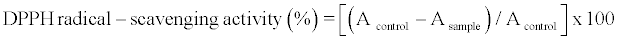
where A is the absorbance at 515 nm.
ABTS•+ assay (TEAC)
The ability of the samples extract to scavenge the ABTS•+ radical was determined using the trolox equivalent antioxidant capacity (TEAC) assay. The method modified by Rufino et al. [29] was used. Briefly, ABTS•+ radical cations were produced by reacting 7 mM ABTS stock solution with 145 mM potassium persulfate and allowing the mixture to stand in the dark at room temperature for 12 h before use. The ABTS•+ solution was diluted with ethanol to an absorbance of 0.800 ± 0.002 at 734 nm. After addition of 20 μl of the sample extract or trolox standard to 4 ml of diluted ABTS•+ solution, absorbance was recorded after 6 min of mixing. Ethanolic solutions of known trolox concentrations (0- 10 μg per ml) were used for calibration (R2= 0.9999) and results were expressed as μmol trolox per 100 g dry sample.
Statistical analysis
Data were expressed as means ± standard deviation (SD) of three replications, and one factor ANOVA was used for the statistical analysis using SPSS program (version 17.0 SPSS Inc).. The values were considered to be significantly different when P<0.05.
Purslane is a reasonable choice due to its high nutritive and antioxidant properties as human food and medical utilization. The effects of three drying methods (hot-air, microwave and freeze drying) on color, some phytochemicals and antioxidant capacity of purslane leaves were as follows:
Color assessment
The color of the food surface is the first quality parameter evaluated by consumers and is critical in product acceptance, even before it is tasted. The chromatic coordinates L* (brightness/ darkness), a* (rednessgreenness) and b* (yellowness-blueness) have been widely used to describe color changes during thermal processing of food products; they have been related to the types and quantities of some components present in those products [30]. The mean color values of fresh and dried purslane leaves are shown in Table 1. Values of fresh purslane leaves L*, a* and b* were 35.90, -4.89 and 9.51, respectively. Results in Table 1 showed that there were statistically significant effects of drying methods on the color of the samples (p<0.05). All drying methods increased brightness (L*), which indicated that dried leaves had a bright color as compared with fresh samples. Whereas, freeze-dried leaves presented significant higher lightness, yellowness and lower a* values than that for hot-air and microwave dried leaves. Therefore the microwave dried leaves were characterized by a more dark and green-bluish color, while higher luminosity and green-yellowish color was in freeze-dried leaves. Martínez-Las Heras et al. [24] found that the infusions obtained from freeze-dried persimmon leaves were more luminosity and greenyellowish color than that from air dried leaves. Dwivedy et al. [31] found that after microwave drying of Indian Borage leaves all the three chromatic coordinates values decreased, but the reduction in L* values was not significant. Though –a* values reduced significantly, still these were within the greenness range and the same was with the +b* values. In hot-air dried leaves, drying temperature significantly induced the increase of a* values from -0.41 (at 50ºC) to -0.04 (at 70ºC). This may be due to non-enzymatic browning reaction, which turned the samples less greenish when the temperature rose [32].
| Drying methods | L* | a* | b* | ΔE |
|---|---|---|---|---|
| Fresh | 35.90d | -4.89a | 9.51b | - |
| 50°C | 52.90b | -0.41c | 9.54b | 17.58b |
| 60°C | 52.95b | -0.36c | 8.12c | 17.70b |
| 70°C | 53.05b | -0.04e | 9.39b | 17.82b |
| 360 W | 49.54c | -0.38c | 7.62c | 14.49c |
| 900 W | 49.64c | -0.16d | 7.24c | 14.71c |
| 1250 W | 52.40b | -0.35c | 10.07b | 17.12b |
| FD | 57.07a | -2.45b | 12.63a | 21.54a |
Values are means of ten replicates
Means within a column marked with different letters are significantly different at
(p < 0.05)
Table 1: Effect of drying methods on chromatic coordinates (L*, a* and b*) and ΔE of fresh and dried purslane leaves samples.
Since ΔE is a function of the three chromatic coordinates, when analyzing the ΔE values, the highest value was observed in freezedried samples (Table 1), as compared with the rest of the treatments (p<0.05). This may be due to that the freeze-dried leaves were more brightness. As the hot-air drying temperature and microwave output power increased, the ΔE values of dried samples increased. This may be because of the effect of high temperature on heat-sensitive components, such as proteins and carbohydrates [33]. The color changes caused by the thermal treatment may be caused not only by the non-enzymatic browning reaction, but also by the destruction of pigments present in the foods [34]
Pigments
Fresh purslane leaves contained 50.75 ± 3.94, 19.12 ± 0.88 and 16.53 ± 0.81 mg/ 100 g (dry weight) of chlorophylls a, b, and β-carotene, respectively (Figure 1). All studied drying methods caused significant increase in pigments contents of purslane leaves. This result could be related to an increase in the extractability of such compounds as a consequence of the matrix changes during the drying process [24]. The freeze-dried purslane leaves had the highest concentrations of chlorophylls a and b. This because of freezing could lead to the development of ice crystal within tissue matrix. Ice crystals could result in a greeter rupturing of cell structure, which may lead to better solvent access and extraction [35]. Some studies concluded that freeze-drying increases the extraction of bioactive compounds of different products in comparison to air drying [21-23]. This is because freeze-drying is based on the dehydration by sublimation of a frozen product [17].
Elevated temperature (during hot-air and microwave drying) led to increase of chlorophylls contents of dried purslane leaves compared to fresh one. A degrading effect was on decreasing of β-carotene content. This could be attributed to oxidation with air. Reports on the effects of microwaves on carotenoids are contradictories. Some authors reported losses of total carotenoids in broccoli during conventional and microwave cooking [36]. Other authors pointed microwave as less destructive cooking method. Howard et al. [37] reported minimal effects on trans-β-carotene in broccoli, carrots or green beans while Mayeaux et al. [38] found that lycopene in tomato was highly affected since about 35.6% of lycopene lost after 1 min of microwave heating at high power.
Total phenolics content and flavonoids
Total phenolics and total flavonoids contents for fresh purslane leaves were found to be 1447.59 mg GAE and 5011.87 mg QE per 100 g (dry weight) sample, respectively. The results in Figure 2 showed that the degradation of total phenolics and flavonoids significantly varied according to the drying methods. It was noted that, extracts of dried leaves always showed lower concentration of total phenolics and flavonoids than those from fresh leaves. The loss of phenolics and flavonoids during drying might be due to the process conditions, in particular the temperatures and the duration used [39,40]. Davey et al. [41] reported that thermal processing can affect the phytochemicals by thermal breakdown that affect the integrity of the cell structure which then resulted in the migration of components, leading to losses by leakage or breakdown by various chemical reactions involving enzymes, light and oxygen. The loss of phenolics and flavonoids as expected was found to be lower with hot-air drying at 50ºC (33.75% and 1.07%, respectively) and freeze-drying (36.18% and 5.22%, respectively) than the other drying treatments set. Lyophilization is often considered to be the most adequate drying technique for preserving temperature sensitive compounds. In the case of our study on purslane leaves, it would not be a competitive process for preserving phenolics and flavonoids since similar or better results were obtained with air drying at 50ºC. Martínez-Las Heras et al. [24] showed a great influence of the drying method on the extraction of phenols during infusion. The extracts from persimmon leaves dried with air at 100ºC gave the highest total phenolics concentration, followed by extracts from lyophilized leaves, and then the extracts from air dried leaves at 180ºC and from shade dried leaves. Zhang et al. [42] found that the oven dried (60ºC/ 16 h) bitter melon leaves lost about 21.5% to 33.2 and 44.4% to 65.9% of total flavonoids and total phenolics, respectively, while in the freezedried product, the loss of total flavonoids and total phenolics was 0.2% to 17.8% and 4.3% to 9.7%, respectively, compared to the fresh product. The same trend is observed in this study. According to Mrad et al. [32], the decrease in total phenolics content during drying can be attributed to the binding of polyphenols with other compounds (proteins) or to alterations in the chemical structure of polyphenols which cannot be extracted or determined by available methods. de Ancos et al. [43] suggested that polyphenolics compounds may be deteriorated depending upon many factors other than heat treatment. These included the activity of polyphenol oxidase, organic acid content, sugar concentration and pH.
It can observed from Figure 2 that the increase in hot-air drying temperature and microwave output power not significantly increased the total phenolics and flavonoids contents of dried purslane leaves. The formation of these compounds at high temperatures might be because of the availability of precursors of these molecules by non-enzymatic inter-conversion between molecules [44].
Regarding the effect of microwave drying, results in Figure 2 showed that the microwave dried purslane leaves exhibited significantly the lowest total phenolics and total flavonoids contents. The total phenolics and total flavonoids contents varied insignificantly with increasing of microwave output powers. Rabeta and Vithyia [45] found that the microwave drying does not cause any significant changes in total phenolics content of Vitex negundo tea. Crozier et al. [46] reported losses of conjugated quercetin of 64% and 65% in microwaved onions and tomatoes, respectively. Also, Vallejo et al. [47] studied the losses in phenolics compounds in broccoli was submitted to high-pressure boiling, low-pressure boiling, steaming and microwaving. They observed clear disadvantages when microwaving was used noticing losses of 97% in flavonoids.
Antioxidant capacity
The antioxidant capacity of fresh and dried purslane leaves was determined by DPPH and ABTS methodologies. DPPH has been widely used to evaluate the free radical scavenging activity of various antioxidant substances. DPPH is a stable free radical which gives rise to the deep violet color in methanol solution, characterized by an absorption band centered at about 520 nm. In the DPPH assay, the antioxidants which can donate hydrogen are able to reduce stable DPPH radical to the yellow colored, non-radical form of DPPH-H. The different drawbacks and advantages of each one of the available antioxidant capacity assays make it necessary to use different techniques [48,49]. Fresh purslane leaves exhibited values of 53.23% and 147.78 μmol trolox per 100 g dry weight for DPPH and ABTS, respectively. All studied drying methods had significantly adverse effects on antioxidant capacity of purslane leaves (Figures 3 and 4). When comparing dried samples, maximum antioxidant capacity was observed in samples dehydrated by different hot-air drying temperatures and freeze-drying method, with no significant differences (p<0.05) for both DPPH and ABTS methodologies. In addition, the lowest values were observed in samples dried by microwaving at different output powers. This coincides with the contents of total phenolics and flavonoids. Chan et al. [6] found that E elatior leaves microwave-dried for 2, 4, 6 and 8 min showed significant declines in ascorbic acid equivalent antioxidant capacity (AEAC) and ferric-reducing power (FRP). Freeze-drying led to slight decline of 9% and 14% for C longa leaves and 10% and 14% for K galangal leaves in AEAC and FRP, respectively. Freeze-dried yam flours and water hyacinth leaves displayed the highest antioxidant activity compared to sun-, hot air- and drum dried ones [50,51]. In general, the generation and accumulation of compounds with a varying degree of antioxidant activity during food dehydration could develop antagonistic or synergistic effects between themselves or with the other sample constituents. These complex chemical interactions that influence functional properties of food during drying are still being researched [34,52,53]
Antioxidant capacity of purslane leaves may be related to the amount of pigments, total phenolics and flavonoids. Since these compounds act as scavengers of the free radicals produced during oxidation reactions. In order to explore the influence of the phytochemical compounds on purslane leaves antioxidants capacity, linear correlation coefficient was determined between the antioxidant capacity and the main antioxidants studied. The higher correlation for DPPH and ABTS was in order total phenolics (R2= 0.9043-0.9885) > total flavonoids (R2= 0.4993-0.6068)>chlorophyll a (R2=0.3791-0.4086)>β-carotene (R2=0.0664-0.0793)>chlorophyll b (R2=0.0219-0.0601). Increasing correlation between antioxidant activity and total phenolics content has been reported during food dehydration [54,55]. Based on our results, total phenolics and flavonoids are useful predictors of antioxidant capacity of purslane leaves. Thus, purslane leaves could be considered as an important source of biologically active components with high antioxidant capacity.
The results in this study are essential in order to obtain the optimum benefits of bioactive compounds present in purslane leaves during drying. According to its high phytochemicals content and antioxidant capacity purslane is considered to be a potential dried product. All studied drying methods (hot-air, microwave, freezedrying) had adverse effects on total phenolics and flavonoids contents and antioxidant capacity of purslane leaves. Hot-air drying and freezedrying had the lowest negative effects. Microwave drying had a clear negative effect on the antioxidant capacity of purslane leaves. Based on this study, a high correlation was observed between total phenolics content with purslane leaves antioxidant capacity determined by DPPH and ABTS assays. Thus, this plant could be considered as an important source of biologically active components with high antioxidant activity to assess the requirements of today’s consumers, who are very interested in the potential role of functional or nutraceutical foods.