Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2017) Volume 7, Issue 5
Probiotics are microorganisms that provide health benefits when consumed. The term probiotic is currently used to indicate the ingested microorganisms associated with beneficial effects to humans and animals. Probiotic drinks are typically dairy-based beverages with consistency similar to milk. They are consumed for digestive health. The addition of probiotics to whey will enhance the properties and benefits to several folds. Addition of orange juice gives it better sensory properties and mainly increases the shelf life of the drink or beverage. The overall goal of the research work was to develop a probiotic beverage from whey and orange juice, to determine the physicochemical properties and to check the shelf-life stability of the juice. The probiotic beverage was prepared by blending whey and orange in five different ratios viz. 80:20, 75:25, 70:30, 65:35 and 50:50. The different blends were inoculated by 1% inoculum of Lactobacillus acidophilus and Bifidobacterium bifidium and the fermentation time was optimized for 24 hours. The 65:35 blend ratios of whey and orange juice fermented for 24 hr gave desirable results with highest sensory scores for overall acceptability and maximum viable counts in both fermented with Lactobacillus acidophilus and Bifidobacterium bifidium. There was no significant difference in results fermented with Lactobacillus acidophilus and Bifidobacterium bifidium but the results of probiotic beverage with Bifidobacterium bifidium showed a little better result as comparison to probiotic beverage fermented with Lactobacillus acidophilus.
Keywords: Lactobacillus acidophilus; Bifidobacterium bifidium; Sensory quality; Storage stability
Foods which promote health beyond providing basic nutrition are termed as functional foods. These foods have the potential to promote health in ways which was not predicted by traditional nutritional science. The general category of functional foods includes processed foods or foods fortified with health-promoting additives, like «vitamin-enriched » products. Functional foods are part of the continuum of products that individuals may consume to increase their health and/or contribute to reducing their disease burden.
The World Health Organization’s 2001 definition of probiotics is «live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host». This means that the microorganisms must be alive and present in high numbers, generally more than 109 cells per daily ingested dose. Each product should indicate the minimum daily amount required for it to confer specific health benefits.
Almeida et al. [1] defined the term “probiotic”, when he suggested the term to denote all organic and inorganic food complexes as “probiotics,” in contrast to harmful antibiotics, for the purpose of upgrading such food complexes as supplements. Bastani et al. [2] Vergio, in his publication Anti-und Probiotika, compared the detrimental effects of antibiotics and other antimicrobial substances with favorable factors (“Probiotika”) on the gut microbiology.
Traditionally, probiotics have successfully been added to a wide range of dairy based food products. However, the problem of lactose intolerance and cholesterol content has increased the demand for non-dairy based probiotic products. About 5-15% of the Europe population is lactose intolerant and this number increased up to 80% in some part of the world such as central Asia and Africa. Dairy products with probiotic bacteria are unsuitable for this group of population because of its health condition. Additionally, with growing awareness of gut healthy, consumers demand a wider variety of probiotic products beyond dairy based food products. This work is a part of an on-going project to evaluate the potential of Brassica vegetables for the development of a probiotic-based product Khurana and Kanawjia [3].
Whey or milk serum is the liquid remaining after milk has been curdled and strained. It is a by-product in the process of cheese production. Composition and characteristics of whey are depending on the production technology of the end product and on the quality of the used milk. Liquid whey consists approximately 93% of water and contains almost 50% of total solids present in the milk of which lactose is the main constituent. Orange (specifically, the sweet orange) is the fruit of the citrus species Citrus sinensis. The fruit of the Citrus sinensis is considered a sweet orange. It is known for its health benefits, particularly its high concentration of vitamin C. Orange juices contain flavonoids that may have health benefits. Orange juice is also a source of the antioxidant hesperidin. Because of its citric acid content, orange juice is acidic, with a typical pH of around 3.5.
Bifidobacterium, along with Lactobacillus, are some of the most well-known probiotics and are an important part of probiotic flora. These good bacteria are Lactic-Acid Producing Bacteria (LAB), just like Lactobacillus, and they both produce vitamins, bacteriocins (antibacterial chemicals) and antibiotic-like substances. Both have significant health benefits on the digestive and immune systems.
The objectives of the present study were:
1. To develop a probiotic beverage from whey and orange juice.
2. To study the physicochemical properties and sensory characteristics of probiotic beverage.
3. To study the storage stability of probiotic beverage.
Materials required
Raw materials
• Whey
• Orange juice
• Sugar
• Culture
After a detailed literature review on health benefits of probiotics, the freeze dried culture of Lactobacillus acidophilus and Bifidobacterium bifidium was obtained from Laboratory of Microbiology, Department of Microbial and Fermentation Technology, Jacob School of Biotechnology and Bioengineering, SHIATS. Dried pellets were reconstituted in 50 mL MRS (deMan, Rogosa and Sharpe medium) broth and grown over night (14-16 hrs) in an incubator shaker at 110 rpm and 37°C. This was sub cultured and grown overnight again. The culture thus obtained after the second sub-culture was used for further experiments.
Other materials
• Stabilizer
• Preservative
• Emulsifiers
• Sodium hydroxide
• Chemicals used
Chemicals were used for the preparation of Lactobacillus MRS Agar media for the cultivation of lactic acid bacteria.
Methods
Preparation of probiotic beverage: The various blends of whey-orange juice prepared using a constant sugar level of 10%, 80W:20J, 75W:25J, 70W:30J, 65W:35J and 50W:50J. The different blends were then fermented with Lactobacillus acidophilus and Bifidobacterium bifidium. The incubation period was optimized by inoculating whey with 1% (v/v) inoculums of Lactobacillus acidophilus and Bifidobacterium bifidium (1 × 105 CFU/mL) with the addition of orange juice.
Physicochemical analysis: Different parameters such as Titratable acidity, Ascorbic acid content, pH, Total Soluble Solids, Protein content, Total Viable count were examined to study the impact of fermentation time and storage period of the developed probiotic beverage. Organoleptic evaluation was also done.
Statistical analysis: In statistical analysis mean method was applied. The arithmetic mean of the replication value was taken. Then the maximum and minimum values of all the microbial counts, physicochemical values and nutritional values during storage were obtained. The formula of the arithmetic mean used was given in the equation 1:
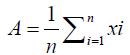 (1)
(1)
The results of the study are being presented and discussed in following section.
Development of probiotic whey-orange beverage
The probiotics whey orange beverage was prepared by blending whey and orange juice in different proportions i.e. T1 (80:20), T2 (75:25), T3 (70:30), T4 (65:35) and T5 (50:50). All the prepared probiotic beverages were then fermented for 24 hrs with Bifidobacterium bifidium and Lactobacillus acidophilus and their viable counts were checked. All prepared samples were then packed in glass bottles and stored at 4°C refrigerated temperature.
Effect of fermentation time on developed probiotic beverage by using Bifidobacterium bifidium and Lactobacillus acidophilus
The viable counts were assessed after 5, 15, 20 and 24 hrs of incubation at 37°C for the probiotic beverage developed fermented with Bifidobacterium bifidium and Lactobacillus acidophilus. As the amount of orange juice increased in the probiotic beverages there was a decrease in total viable counts. But in each probiotic beverage fermented with Bifidobacterium bifidium and Lactobacillus acidophilus the total viable count increased significantly with increase in fermentation time from 5 to 24 h. Both whey and whey orange juice probiotic beverage attained a total viable count of more than 106 cfu/ml within 24 hrs of fermentation. The results were shown in the Table 1. As the 24 hrs fermentation showed maximum viable counts all the probiotic beverages were fermented for 24 hrs for further analysis.
| Fermentation time (h) | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| 5 | 0.24 | 0.47 | 0.43 | 0.42 | 0.39 | 0.36 |
| 15 | 4.02 | 5.76 | 5.64 | 5.44 | 5.34 | 5.07 |
| 20 | 7.18 | 7.19 | 7.13 | 6.98 | 6.88 | 6.49 |
| 24 | 8.03 | 8.58 | 8.47 | 8.32 | 8.29 | 8.12 |
Table 1: Effect of fermentation time on developed probiotic beverage by using Bifidobacterium bifidium (colonies in 108).
The stress induced may be due to the differences between the pre-culture and the fermentation medium resulted in decrease of the growth rate at the earlier stage of fermentation process. MRS broth, as the pre-culture, has a pH of about 5.6 but the initial pH of the whey orange juice was relatively lower (about 4.0). Holzapfel et al. [4] showed similar results.
Effect of fermentation on physicochemical properties of developed probiotic beverages by using Lactobacillus acidophilus and Bifidobacterium bifidium
The probiotic beverage developed fermented with Bifidobacterium bifidium and Lactobacillus acidophilus was fermented for 24 hrs. Then the physicochemical properties of each probiotic beverage were determined. The pH of the different probiotic beverages decreased as the amount of orange increased in the probiotic beverages. Almeida et al. studied the effects of the different combinations of Lactobacillus delbrueckii subsp. bulgaricus (Lb), Lactobacillus acidophilus (La), Lactobacillus rhamnosus (Lr), and Bifidobacterium animalis sub sp. lactis (Bl) in co-culture with Streptococcus thermophilus (St) on the rate of acid development in milk and milk-whey mixture, and (b) the effect of the level of the total solids of the different bases on the acidification profile and viability of potential health-promoting microorganisms. The co-culture of St-Lr showed the lowest values Vmax in all bases; while the co-culture St-Bl had high Vmax in milk and whey bases (12 and 10 g/100 g, respectively). Co-cultures St-La and St-Lb reached Vmax at pH 5.5, while St-Lr and St-Bl at pH 5.91. The pH of the different probiotic beverages T1, T2, T3, T4 and T5 fermented with Bifidobacterium bifidium ranged from 3.89 to 3.34. The results were shown in the Table 2 [5]. The pH of similar probiotic beverages fermented with Lactobacillus acidophilus ranged from 3.85 to 3.33. Higher viable counts during the initial period of fermentation resulted in comparative lowering of pH for whey fermented along with orange juice. The result was supported by Shukla et al. [6]. The TSS (Total soluble solids) of the different probiotic beverages fermented with Bifidobacterium bifidium ranged from 14.4 to 12, the results were shown in the Table 3 and that of probiotic beverages fermented with Lactobacillus acidophius ranged from 14.37 to 11.9. The results were shown in the Table 4. The TSS of the probiotic beverage mainly depends upon the whey protein and sugar. The TSS of the probiotic beverage decreases as the amount of orange juice increases in the beverage. The titratable acidity of the probiotic beverages fermented with Bifidobacterium bifidium ranged from 0.897 to 1.008, the results were shown in the Table 3 and that of probiotic beverages fermented with Lactobacillus acidophilus ranged from 0.895 to 1.005. The results were shown in the Table 4. As the consequence of fermentation process a variation of the fermentable sugars and an accumulation of acidity are to be expected. The ascorbic acid content in mg/100 ml of the probiotic beverages fermented with Bifidobacterium bifidium ranged from 38.52 to 52.31, the results were shown in the Table 3 and that of probiotic beverages fermented with Lactobacillus acidophilus ranged from 38.49 to 52.29. The results were shown in the Table 4. The protein content of the probiotic beverages fermented with Bifidobacterium bifidium ranged from 0.51 to 0.19, the results were shown in the Table 3 and that of probiotic beverages fermented with Lactobacillus acidophilus ranged from 0.49 to 0.17. The results were shown in the Table 4. The protein content of the probiotic beverages decreases as the whey proteins are more susceptible to heat treatment are denatured at temperature of 70°C and above with the reduction in pH. Prendergast [5] stated the same results.
| Fermentation time (h) | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| 5 | 0.22 | 0.47 | 0.43 | 0.42 | 0.39 | 0.36 |
| 15 | 6.79 | 5.73 | 5.59 | 5.41 | 5.30 | 5.02 |
| 20 | 9.24 | 7.16 | 7.03 | 6.92 | 6.83 | 6.45 |
| 24 | 9.79 | 8.54 | 8.43 | 8.3 | 8.25 | 8.06 |
Table 2: Effect of fermentation time on developed probiotic beverage by using Lactobacillus acidophilus (colonies in 108).
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| pH | 4.6 | 3.89 | 3.71 | 3.62 | 3.53 | 3.34 |
| TSS (°Brix) | 16.3 | 14.4 | 13.9 | 13.1 | 12.8 | 12 |
| TA (%) | 1.353 | 0.897 | 0.926 | 0.954 | 0.988 | 1.008 |
| Ascorbic Acid (mg/100 ml) | 0 | 38.52 | 40.96 | 43.62 | 45.27 | 52.31 |
| Protein (%) | 0.7 | 0.51 | 0.42 | 0.35 | 0.28 | 0.19 |
Table 3: Effect of fermentation on physicochemical properties of developed probiotic beverages by using Bifidobacterium bifidum.
| Parameters | T0 | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|---|
| pH | 4.6 | 3.85 | 3.69 | 3.6 | 3.51 | 3.33 |
| TSS (°Brix) | 16.3 | 14.37 | 13.88 | 12.9 | 12.7 | 11.9 |
| TA (%) | 1.351 | 0.895 | 0.923 | 0.951 | 0.982 | 1.005 |
| Ascorbic acid (mg/100 ml) | 0 | 38.49 | 40.92 | 43.59 | 45.25 | 52.29 |
| Protein (%) | 0.7 | 0.49 | 0.4 | 0.33 | 0.26 | 0.17 |
Table 4: Effect of fermentation on physicochemical properties of developed probiotic beverage by using Lactobacillus acidophilus.
Effect of fermentation on organoleptic properties of developed probiotic beverage by using Bifidobacterium bifidium and Lactobacillus acidophilus
In all the probiotic beverages fermented with Bifidobacterium bifidium and Lactobacillus acidophilus the points representing the average score of its different parameters like color, taste, aroma and overall acceptability have been presented in the Tables 5 and 6. The results revealed that colour, taste aroma and overall acceptability of the probiotic beverage 4 (65:35) gets the best result in both fermented with Bifidobacterium bifidium and Lactobacillus acidophilus.
| Parameters | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Colour and appearance | 6.31 | 6.93 | 7.18 | 7.93 | 6.48 |
| Taste | 6.34 | 6.89 | 7.19 | 7.51 | 6.74 |
| Consistency | 6.37 | 6.87 | 7.25 | 7.81 | 7.89 |
| Flavour | 6.18 | 6.75 | 7.37 | 8.37 | 7.23 |
| Overall Acceptability | 6.3 | 6.86 | 7.24 | 7.9 | 7.08 |
| Colour and appearance | 6.28 | 6.91 | 7.15 | 7.91 | 6.45 |
| Taste | 6.31 | 6.86 | 7.14 | 7.48 | 6.71 |
Table 5: Effect of fermentation on organoleptic properties of developed probiotic beverage by using Bifidobacterium bifidium.
| Parameters | T1 | T2 | T3 | T4 | T5 |
|---|---|---|---|---|---|
| Colour and appearance | 6.28 | 6.91 | 7.15 | 7.91 | 6.45 |
| Taste | 6.31 | 6.86 | 7.14 | 7.48 | 6.71 |
| Consistency | 6.35 | 6.84 | 7.21 | 7.77 | 7.85 |
| Flavour | 6.15 | 6.73 | 7.34 | 8.35 | 7.21 |
| Overall acceptability | 6.27 | 6.83 | 7.21 | 7.87 | 7.05 |
Table 6: Effect of fermentation on organoleptic properties of developed probiotic beverage by using Lactobacillus acidophilus.
The results above declares from physicochemical and sensory evaluation, that only probiotic beverage T4 (65:35) has shown best results from both the bacterial species fermented samples. Therefore for shelf life study only probiotic beverage 4 (65:35) has been taken.
Effect of storage period on total viable count and acidity of developed probiotic beverage (65:35) by using Bifidobacterium bifidium and Lactobacillus acidophilus
The developed probiotic beverage T4 (65:35) fermented with Bifidobacterium bifidium and Lactobacillus acidophilus was packed in glass bottles and were stored at 4 ± 1°C. The juice was evaluated for total viable counts and nutritional composition after every 15 days. The total viable counts in the probiotic beverage fermented with Bifidobacterium bifidium and Lactobacillus acidophilus decreased from 8.29 × 108 to 5.8 × 107 and 8.25 × 108 to 5.2 × 107 respectively. The results were shown in Tables 7 and 8 respectively. There was nominal change found in acidity on storing the samples for 45 days in both probiotic beverages fermented with Bifidobacterium bifidium and Lactobacillus acidophilus. The decrease in viable count is due to the decrease in the pH of the medium and accumulation of organic acid as a result of growth and fermentation. As the pH of the medium decreased the titratable acidity increased.
| Storage period (in days) | Viable count (cfu/ml) | Acidity (%) | Protein (%) | Ascorbic acid (mg/100 ml) |
|---|---|---|---|---|
| 0 | 8.29 | 0.926 | 0.28 | 45.27 |
| 15 | 6.62 | 0.929 | 0.26 | 44.43 |
| 30 | 5.17 | 0.932 | 0.25 | 43.65 |
| 45 | 0.58 | 0.941 | 0.24 | 42.17 |
Table 7: Effect of storage period on developed probiotic beverage T4 (65:35) by using Bifidobacterium bifidium.
| Storage period (in days) | Viable count (cfu/ml) | Acidity (%) | Protein (%) | Ascorbic acid (mg/100 ml) |
|---|---|---|---|---|
| 0 | 8.25 | 0.923 | 0.26 | 45.25 |
| 15 | 6.57 | 0.928 | 0.24 | 44.41 |
| 30 | 5.13 | 0.936 | 0.23 | 43.63 |
| 45 | 0.52 | 0.953 | 0.21 | 42.14 |
Table 8: Effect of storage period on developed probiotic beverage T4 (65:35) by using Lactobacillus acidophilus.
In nutritional composition the ascorbic acid content decreased from 45.27 (mg/100 ml) to 42.17 (mg/100 ml) in probiotic beverage fermented with Bifidobacterium bifidium and from 45.25 (mg/100 ml) to 42.14 (mg/100 ml) in probiotic beverage fermented with Lactobacillus acidophilus. The results were shown in Tables 7 and 8. It was observed that there was a slight decrease in ascorbic acid content during 45 days of storage period. This may be due to pasteurization of juice and light exposure. There were nominal changes in protein content.
Effect of storage period on viable count of yeast and moulds of developed probiotic beverage (65:35)
Some species of microorganisms grow in food products with low pH, but results show that there were no microbial activity including acidophilic bacteria, lactic acid bacteria, yeast and mold over a period of 45 days at 4°C in the samples of fruit juice beverages. The microbial analysis was done by the standard plate count for yeast and moulds. The SPC was done in duplicates and was analyzed for 45 days of storage at every 15 days of interval. The results shown in Table 9 depicts that the growth in the product at 105 dilutions was nil. Thus it was fit for further storage.
| Storage period (in days) | 10-Jan | 10-Feb | 10-Mar | 10-Apr | 10-May |
|---|---|---|---|---|---|
| 0 | 0.53 | - | - | - | - |
| 15 | 0.59 | - | - | - | - |
| 30 | 1.09 | 64 | 11000 | - | - |
| 45 | 1.2 | 108 | 58000 | - | - |
| 60 | 2.08 | 185 | 67000 | - | - |
Table 9: Effect of storage period on viable count of yeast and moulds colonies (in 103) of developed probiotic beverage (65:35).
The physicochemical analysis was done for different blends both fermented with Bifidobacterium bifidium and Lactobacillus acidophilus. The pH of the different treatments fermented with Bifidobacterium bifidium ranged from 3.89 to 3.34 and that of treatments fermented with Lactobacillus acidophilus ranged from 3.85 to 3.33. The TSS ranged from 14.4 to 12 of treatments with Bifidobacterium bifidium and from 14.37 to 11.9 fermented with Lactobacillus acidophilus. The titratable acidity of the treatments ranged from 1.008 to 0.897 and from 1.005 to 0.895 in produced probiotic beverage fermented with Bifidobacterium bifidium and Lactobacillus acidophilus respectively. The ascorbic acid content of the treatments ranged from 38.52 to 52.31 and from 38.49 to 52.29 in produced probiotic beverage fermented with Bifidobacterium bifidium and Lactobacillus acidophilus respectively. The protein content of the blends fermented with Bifidobacterium bifidium ranged from 0.51 to 0.19 and the blends fermented with Lactobacillus acidophilus ranged from 0.49 to 0.17. According to sensory analysis it was found that out of 5 blends Blend 4 with whey and orange in ratio (65:35) was most widely accepted in comparison to other treatments in both fermented with Bifidobacterium bifidium and Lactobacillus acidophilus. So this blend was used for further analysis.
The total viable counts and nutritional composition was evaluated at every 15 days interval for 45 days. The total viable counts in the blend fermented with Bifidobacterium bifidium decreased from 8.29 × 108 to 5.8 × 107 and Lactobacillus acidophilus decreased from 8.25 × 108 to 5.2 × 107. In nutritional composition the ascorbic acid content decreased from 45.27 (mg/100 ml) to 42.17 (mg/100 ml) in blend fermented with Bifidobacterium bifidium and from 45.25 (mg/100 ml) to 42.14 (mg/100 ml) in blend fermented with Lactobacillus acidophilus. This may be due to pasteurization of juice and light exposure. The shelf life study of the prepared probiotic juice was studied. It was found that the microbial count was acceptable and yeast and mold count was nil which showed that the product could be stored for 45 days.
Study revealed satisfactorily good quality probiotic beverage with therapeutic value prepared by using a 63:35 blend of whey and orange juice inoculated with 1% (v/v) of Lactobacillus acidophilus and Bifidobacterium bifidium and incubated for 24 hours with a shelf life of 45 days. The microbial count found was 8.29 × 108 cfu/ ml in Bifidobacterium bifidium fermented beverage and 8.25 × 108 cfu/ml in Lactobacillus acidophilus fermented beverage. The values of pH, TSS, TA, Ascorbic acid content and Protein determined were 3.62, 13.1°Brix, 0.926%, 45.27 mg/100 ml and 0.28% respectively in beverage fermented with Bifidobacterium bifidium. The values of pH, TSS, TA, Ascorbic acid content and Protein determined were 3.51, 12.7°Brix, 0.923%, 45.25 mg/100 ml and 0.26% respectively in beverage fermented with Lactobacillus acidophilus. It also depicted that whey orange blend serves as a good fruit matrix for the fermentation of Lactobacillus acidophilus and Bifidobacterium bifidium. The results also showed that the beverage fermented with Bifidobacterium bifidium had better properties than the same blend fermented with Lactobacillus acidophilus. On the basis of above results revealed in the present study it might be concluded that the formulated probiotic juice beverage was possible to satisfy consumer taste and preferences.