Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2017) Volume 7, Issue 5
The aim of his study was to prepare ham pâté with added kiwi fruit skin (Actinidia deliciosa) to evaluate its effect as a natural antioxidant, to check the stability of the pâté during storage, and to assess its acceptability. The treatments were as follows: SHP-standard ham pâté, with sodium erythorbate; CHP-control ham pâté, without antioxidant; KHP-I-ham pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II-ham pâté with 1.0% KSF and KHP-III-ham pâté with 2.0% KSF. The following analyses were performed: Chemical composition, pH, color, lipid oxidation, antioxidant activity, vitamin C, texture, as well as microbiological and sensory analysis. The treatments KHP-II and KHP-III could be considered as sources of dietary fiber. The lipid oxidation and microbiological count remained stable. Sensory analysis showed that the treatments were well accepted, except KHP-III. The use of 1.0% KSF proved to be feasible as a natural antioxidant and a source of dietary fiber without changing the quality of the pâtés.
Keywords: Ham pâté; Lipid oxidation; Kiwi fruit; Skin flour
Most previous studies have applied extracts from fruits, skin or seeds as a natural antioxidant in meat products; however, this study aimed to apply kiwi fruit skin flour as a natural antioxidant, thereby using a residue which has antioxidant activity and provides dietary fiber. The addition of the flour instead of the extract reduces the costs related to the extraction of compounds, such as specific reagents and equipment, increasing profit for industrial purposes. Meat does not contain fiber; however we consider that developing a meat product that is rich in fiber, with antioxidant activity, and which can bring benefits to humans from a by-product is of paramount importance. We believe that the merit of this study is that it prepared a healthy meat product, which was rich in fiber and with natural antioxidant properties, from a by-product.
Lipid oxidation is one of the main factors that affect the quality of meat and meat products; it defines the shelf life of products in that it generates undesirable effects from the sensory and nutritional points of view, and it results in the formation of toxic substances [1]. Antioxidants prevent or significantly retard lipid oxidation and can be classified as natural or synthetic [2].
Synthetic antioxidants, such as sodium erythorbate and sodium ascorbate, are most used in meat products due to their effectiveness, low cost and contribution to the color of products. However, consumers are increasingly aware of the toxicological implications of these additives and research has shown that high doses may promote carcinogenic and mutagenic effects [3].
Natural antioxidants are found in plants, bacteria and fungi. They are generally non-toxic and rarely cause adverse side effects. Fruits have been reported as a source of bioactive compounds and many of them contain antioxidant activity. These bioactive compounds include the class of phenols, phenolic acids, flavonoids, tocopherols, phytic acid, ascorbic acid, pigments, and sterols [4].
Kiwi fruits (Actinidia deliciosa) originated in Asia and they are now found worldwide due to their characteristic flavor and significant nutritional value. This fruit is marketed mainly in natura but there are also several products on the market based on kiwi fruit such as pulp, juices, jellies and ice cream. This manufacturing generates waste, which is largely discarded. However, it could be used as food ingredients, since it is rich in minerals, fiber, vitamin C, phenolic compounds, and other phytochemicals [5].
The evolution of agribusiness and the development of food processing processes have led to the generation of large quantities of waste, which is a major environmental problem worldwide [6]. A large proportion of fruit production is currently directed to meet the demand for fresh fruit; however, there is also a global trend for processed products such as preserves, juices, jellies and candies. The manufacture of fruit juice generates large amounts of industrial waste, which is mainly discarded (85%) or used for animal feed in the form of a component in mixed feed or green manure (about 15%), procedures that lead to economic and biotechnological losses for the food industry [7]. The growing market for natural products, coupled with consumer interest in disease prevention, has prompted the food industry to search for healthier products, and consequently there has been much research in this area. The idea of traditional foods with added nutrients in their formulation is a practice that provides access to nutrients for consumers, without requiring the need to change eating habits [8]. Changes in processing, and growing consumer demand for foods with sensory and nutritional quality that bring health benefits, have encouraged the study of new ingredients for the food industry [9].
The aim of this study was to prepare ham pâté with added kiwi fruit skin flour (Actinidia deliciosa) and to assess its quality, analyzing the effect of the flour as a natural antioxidant, checking the stability of the pâté during storage and its acceptability.
Raw materials
Ingredients: The boneless pork, ham, lard and bacon were provided by a refrigerated store located in Santa Cruz do Sul, Rio Grande do Sul. The kiwi fruit (Bruno variety) were obtained from a fruit distributor in Farroupilha, Rio Grande do Sul and the remaining ingredients were purchased in commercial establishments in the city of Santa Maria, Rio Grande do Sul.
Kiwi fruit skin flour: Bruno variety kiwi fruit were used to obtain the KSF. These were at an early stage of maturation (9° Brix) and were peeled by hand using stainless steel knives. The skins were distributed in aluminum forms and were dried at 35+5°C in an air circulating oven for 72 hrs. After drying, the skins were ground in a refrigerated analytical mill (4°C) (Quimis model Q 298ª21, Diadema, Brazil), and the particle size was standardized using 38 mesh sieves of 0.5 mm diameter. The ground material was then vacuum packed and stored in a freezer (-18°C) until use.
Determination of total phenolic compounds: In order to estimate the total phenolic compounds, the methodology described by Singleton et al. [9] was used. The extracts were diluted in a volumetric flask in 80% ethanol at a ratio of 1:50 (v/v) for the flour made from kiwi fruit skin and 1:25 (v/v) for the flour made from bagasse. Subsequently, an aliquot (0.2 mL) of solution was mixed with 1 mL of 2N Folin-Ciocalteu reagent (diluted 1:10). After being kept for 8 min in the dark 0.8 mL of 7.5% sodium carbonate solution (Na2CO3) was added. After incubation at 25°C for 2 h, the absorbance was measured at 765 nm using a spectrophotometer (SP-220 Biospectro brand, São Paulo, Brazil). A standard curve was performed for quantification (y=0.0115x+0.0151, R2=0.9988) using gallic acid in concentrations of 0 to 70 mg.L-1. The values were expressed in mg of gallic acid in 100 g of flour.
Antioxidant activity-2,2-diphenyl-1-picrylhydrazyl–DPPH: The technique described by Brand-Williams et al. was used, which consists of the incubation (30 min) of 5 mL of a methanol solution of 0.1 mM DPPH with 5 mL of solution containing the following increasing concentrations of the extracts (item 2.2): 0.3; 0.6; 1.25; 2.5; 5.0; 10; 15; 20; 25; 30; 35 and 40 mg/mL. A control solution (methanol solution of 1% DPPH) and a blank (methanol) were prepared simultaneously. After incubation, readings were taken of the samples using a spectrophotometer (SP-220, Biospectro brand, São Paulo, Brazil) at a wavelength of 517 nm. The percentage of Antioxidant Activity (AA%) was calculated using the percentage of capture of the DPPH radical, according to Equation 3.
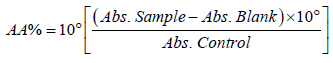 (1)
(1)
Using the DPPH percentage curve (y=2.2533x+2.3831, R2=0.995) versus the concentration of the sample it was possible to obtain the amount of antioxidant required to decrease the initial concentration of DPPH by 50% (IC50) in a fixed reaction time.
Antioxidant activity–FRAP method: The method described by Benzie and Strain was used to determine the iron reducing power of the extracts. The FRAP reagent (Fe (III) solution-TPTZ) was obtained by combining 25 mL of 0.3 M acetate buffer, 2.5 mL of a solution of 10 mM TPTZ (tripyridyltriazine) (3.12 g of TPTZ in 1 L of 40 mM HCl) and 2.5 mL of a 20 mM aqueous ferric chloride solution. In a test tube, 200 uL of sample, which had been pre-diluted in distilled water (at a ratio of 1:50 for the kiwi fruit skin flour and 1:25 for the bagasse flour), and 1.8 mL of FRAP reagent were added together and then kept in a water bath at 37°C for 30 min. The FRAP reagent was used as a blank. The absorbance was measured at 593 nm using a spectrophotometer (SP-220, Biospectro brand, São Paulo, Brazil). The TEAC compound (Trolox equivalent antioxidant capacity) (range of 0 to 25 μM) was used for the calibration curve (Y=0.0601x-0.0679, R2 =0.9937). The results were expressed as μMol TEAC/100 g of flour [10].
Preparation of the products
The preparation of the ham pâtés was performed in the Meat and Meat Products Pilot Plant at the UFSM Polytechnic College and followed the procedures described by Terra [10] taking into account the ingredients cited by the specific legislation [11]. Firstly, the boned meats (pork, pork ham, lard and bacon) were chopped (30 g) with the aid of heavy sterilized knives, and cooked in boiling water (30 min). Then they were ground in an industrial grinder (disk 12) and sent to the cutter, together with the other ingredients (Table 1) for the formation of the meat emulsion.
| Ingredients (%) | SHP | CHP | KHP-I | KHP-II | KHP-III |
|---|---|---|---|---|---|
| Water | 16 | 16 | 16 | 16 | 16 |
| Pork | 44.1 | 44.6 | 44.1 | 43.6 | 42.6 |
| Ham | 10 | 10 | 10 | 10 | 10 |
| Lard | 12 | 12 | 12 | 12 | 12 |
| Bacon | 12 | 12 | 12 | 12 | 12 |
| Salt | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Spirit | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| 10% citric acid | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| Spices (oregano and pepper) | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Isolated soy protein | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| Tripolyphosphate | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Curing salt | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Color fixative | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Sodium erythorbate | 0.5 | - | - | - | - |
| KSF | - | - | 0.5 | 1.0 | 2.0 |
| Carmine dye | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 |
| Total | 100% | ||||
Table 1: Ingredients used in the formulations of ham pâtés made with added synthetic antioxidant and different concentrations of flour made from kiwi fruit skin.
SHP–Standard ham pâté, with sodium erythorbate; CHP-Control ham pâté, without antioxidant; KHP-I–Ham pâté with 0.5% kiwi fruit skin flour (KSF); KHP-II-Ham pâté with 1.0% KSF and KHP-III-Ham pâté with 2.0% KSF.* The curing salt was composed of refined salt, nitrite and sodium nitrate ** The color fixative was sodium ascorbate.
After the formation of the emulsion, the pâtés were stored in 200 mL glass jars; these jars had covers and were impervious to moisture (previously sterilized in boiling water for 20 min). The pâtés were pasteurized to a temperature of 73°C inside the mass. They were then cooled and taken immediately to a refrigerator (4°C) for the whole of the storage period (84 days).
Chemical composition of pâtés
The chemical composition of the pâtés was performed in the laboratories of the Department of Food Science and Technology (UFSM). All the analyses were performed in triplicate and followed the methodology described in the Association of Official Analytical Chemists (AOAC): Moisture (967.03), ash (942.05), crude protein (981.10), ether extract (920.39) and total dietary fiber (985.29). The level of carbohydrates was obtained by difference [12]. The calorific value was calculated by multiplying the results of lipids, proteins and carbohydrates for their caloric values, which were 9, 4 and 4 Kcal, respectively.
Measurement of pH and water activity (aw)
The measurements of pH and water activity (aw) were performed on days 1, 7, 14, 21, 28, 35, 42, 49, 56 and 63. For the determination of pH, the pâté was mixed with distilled water at a ratio of 1:10 [12] and measured using a Digimed® (Model DM-23, São Paulo, Brazil) digital potentiometer. For the analysis of water activity, 3 g of pâté at room temperature (25°C) in specific equipment, Aqua Lab® 4TEV series (Decagon Devices Inc., USA) was used.
Measurement of color
The CIELAB color system was used to measure the color on days 1, 7, 14, 21, 28, 35, 42, 49, 56 and 63, using a Minolta® CM-700d colorimeter (Konica Minolta Sensing Americas Inc., Ramsey, New Jersey, USA). They following variables were measured: L* (brightness), a* which characterizes color in the red region (+a*) to green (-a*), and b* which characterizes the color in the yellow region (+ b*) to blue (-b*). An average value was obtained for 10 readings of each treatment, which were performed in different points of a homogenizer in 3 petri dishes, with a depth of 2.5 cm.
Lipid oxidation–determination of thiobarbituric acid reactive substances-TBARS
TBARS were measured every 7 days until 80 days of storage. The methodology proposed by Raharjo et al. [13] was followed, where 10 g of pâté was weighed and added to 1 mL of 0.15% BHT, 4 mL of 1.5% sulfanilamide and 36 mL of 5% TCA. The mixture was homogenized and filtered through a 50 mL flask, which was supplemented with 5% TCA. Then 2 mL of the mixture in the flasks was mixed with 2 mL of 0.08M TBA and the mixture stood for 80 min at 40°C in a water bath. For the calculation, a standard curve was performed with 1,1,3,3-tetraethoxypropane (TEP), y=1E+07x+0.0034, R2=0.9998. The results were expressed in mg of malonaldehyde (MDA) per kg of sample.
Measurement of antioxidant activity-2,2-difenil-1-picrilhidrazil- DPPH
The measurement of antioxidant activity occurred on days 7 and 60, through the inhibition of the DPPH radical (2,2-diphenyl-1- picrylhydrazyl) using the method reported by Yu et al. [14]; 5 g of pâté was homogenized with 50 mL of 80% ethanol, followed by filtration. The reaction was initiated by transferring 0.5 mL of the filtrate to a test tube containing 3.5 mL of a control solution of methanol-DPPH (0.004 g of DPPH in 100 mL of methanol). After 30 min of incubation in the dark the absorbances were measured at 517 nm using a spectrophotometer. The inhibition of DPPH (%) was calculated according to Equation 2.
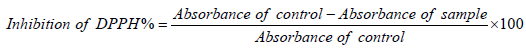 (2)
(2)
Measurement of level of vitamin C
The vitamin C analysis was performed on days 7 and 60 using 5 g of pâté with the addition of 50 mL of 1% oxalic acid. An aliquot of 10 mL was withdrawn from this mixture, which was diluted in 50 mL of 1% oxalic acid and which was titrated using an indicator of 2,6-diclorofenol indophenol. The result was calculated by using Equation 1.
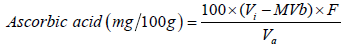 (3)
(3)
Where: Vi: Volume of indophenol solution used in the titration of the sample; Va: Volume of sample used in the titration; MVb: Average volume of indophenol solution used in titration of blank; F: Indophenol solution factor, Vc: Volume of solution used in titration of ascorbic acid.
Microbiological analyses
The microbiological analyses were performed on the first day of the preparation of the pâtés and in order to assess the stability of the pâtés, the mesophilic and psychotropic bacteria were analyzed every 7 days at 4°C for 63 days of storage. The analyses were performed according to RDC No. 12, which is described by ANVISA [15] through the methodologies laid out in the Normative Ruling No. 62 of ANVISA [16].
Portions of 25 g of ham pâté were homogenized with 225 mL of BPW and dilutions in 0.1% peptone water were used for the microbiological analyses. The total coliform counts were performed at 35°C (total) in VRB (violet red bile) agar. The coliforms at 45°C were performed in EC broth (Escherichia coli); coagulase-positive Staphylococcus in Baird- Parker agar (36°C/48 h); Salmonella sp in tetrathionate brilliant green broth and Rappaport-Vassiliadis enrichment broth (42.5°C/24 h), followed by isolation in plates containing SS and Rajhans media. The sulfite-reducing Clostridium count was performed in SPS agar, incubated in anaerobic jars. The total mesophilic aerobic microorganisms count was performed using standard agar culture medium in plates for depth (37°C/48 hrs) and the psychrotrophic bacteria count was performed using standard agar culture media inoculated in surface plates (7°C/7- 10 days ) [16].
Sensory analysis
The sensory analysis was performed in the Sensory Analysis Laboratory of the Department of Food Science and Technology of the Federal University of Santa Maria, Brazil; the analysis was carried out in individual cabins equipped with special lighting. The analysis was performed after microbiological analyses of the product, six days of storage of the pâtés at 4°C. For each sensory analysis test 50 untrained tasters were used, who were randomly recruited at the Rural Science Center of the UFSM. The latter were students, faculty and staff from the Food Science and Technology course. Before analyzing the products the participants received a free and informed consent form that explained the conditions of the sensory tests. The research protocol was approved by the local Research Ethics Committee (CAAE: 26761314.3.0000.5346).
Two sensory methods were used: affective and discriminating. The first method was used to verify the differences of the samples relative to a standard sample and the second method was used to assess the acceptance of the products [17].
For the discriminating analysis, a multiple comparison test was performed; six samples of refrigerated pâté (4°C) together with salty crackers and a glass of water were simultaneously displayed and the standard was identified as such. The other samples were coded, using three random numbers. The tasters compared the standard sample with the other samples by using a seven-point scale. This ranged from extremely lighter, extremely less intense or extremely less firm than the standard (corresponding to the value 1) to extremely darker, extremely stronger and extremely firmer than standard (corresponding to the value of 7). The intermediate value was 4, which was equal to the standard. The evaluated parameters were color, odor, flavor and texture. The tasters took an average of 10 min to evaluate the samples.
For the affective analysis, hedonic scale acceptance tests were used, as well as a purchase intent test. In the first acceptance test the scale was converted into numerical scores which ranged from ‘disliked very much’ (1) to ‘liked very much’ (7). The attributes of color, odor, flavor, texture and overall appearance were evaluated. The samples were distributed in a monadic manner among the testers, together with salty crackers and a glass of water. The purchase intent test was performed by using a closed question as to whether the tester would buy or not buy the product.
Statistical analysis
For the experimental design, each treatment was repeated three times. The analyses were also performed in triplicate. The data were subjected to an Analysis of Variance (ANOVA) by two-way and the mean values were compared using the Tukey test, using a significance level of 5%, for a completely randomized design. For the sensory analysis using the multiple comparison tests, Dunnett’s test was used to compare the means. The data were presented by their mean and standard deviation using Statistica® 9.1 software system [18], which was licensed by the Federal University of Santa Maria.
Chemical composition of pâtés
Table 2 shows that the pâtés were in accordance with the technical regulation of Identity and Quality related to pâtés from the Ministry of Agriculture, which establishes a maximum of 70% moisture, 10% of total carbohydrates, 32% of total fat and at least 8% protein [19].
| Constituents (%) | KSF | SHP | CHP | KHP-I | KHP-II | KHP-III |
|---|---|---|---|---|---|---|
| Moisture | 11.41 ± 0.08 | 63.87a+0.40 | 63.72a+0.16 | 63.98a+0.39 | 60.98b+0.90 | 61.48b+0.35 |
| Proteín | 4.10 ± 0.05 | 17.68ab ± 0.89 | 18.70a ± 0.12 | 17.07b ± 0.64 | 17.25ab ± 0.21 | 16.88b ± 0.44 |
| Ash | 4.33 ± 0.06 | 1.64b ± 0.03 | 1.66ab ± 0.08 | 1.72ab ± 0.07 | 1.74ab ± 0.02 | 1.75a ± 0.03 |
| Lipids | 2.25 ± 0.26 | 15.25c ± 0.14 | 14.34d ± 0.11 | 14.20d ± 0.17 | 16.61a ± 0.15 | 16.12b ± 0.10 |
| Dietary fiber | 28.79 ± 0.30 | 1.46c ± 0.04 | 1.53c ± 0.09 | 2.73b ± 0.25 | 3.01ba ± 0.30 | 3.43a ± 0.16 |
| Carbohydrates* | 49.12 | 0.1 | 0.05 | 0.3 | 0.41 | 0.34 |
| Estimated calorific** | 233.13 | 208.37 | 204.06 | 197.28 | 220.13 | 213.96 |
| Phenolic compounds (mgGAE/100g) | 1262.3 ± 2.7 | - | - | - | - | - |
| IC50 em mg/ml | 6.69 ± 0.60 | - | - | - | - | - |
| FRAP µmol TEAC/100g |
357.5 ± 5.6 | - | - | - | - | - |
Table 2: Chemical composition of Kiwi Fruit Skin Flour (KSF) and of the ham pâtés with different levels of KSF.
a-c Means on the same line with the same superscript letters do not differ significantly by Tukey’s test (p<0.05). Means+standard deviation of analyses in triplicate. KSF-kiwi fruit skin flour. SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control ham pâté, without antioxidant; KHP-I–Ham pâté with 0.5% kiwi fruit skin flour (KSF); KHP-II-Ham pâté with 1.0% KSF and KHP-III-Ham pâté with 2.0% KSF.
*Values obtained by difference. **Kcal/100 g.
The moisture ranged from 60.98 to 63.98%. There was no significant difference between the SHP, CHP and KHP-I pâtés; however, they differed from KHP-II and KHP-III, which showed lower moisture content because of the higher concentration of KSF. This finding in the present study was in accordance with López–Vargas et al. [20], who reported a decrease in moisture content in products with 2.5% added passion fruit residue; the content ranged from 53.13% to 50.72% in terms of moisture.
The amount of protein found in the pâtés ranged from 16.88 to 18.70%. The SHP, KHP-I, KHP-II and KHP-III treatments did not differ between themselves because the KSF contained low amounts of protein (4.10%).
The ash content (Table 2) ranged from 1.64 to 1.75%, with significant difference only between treatments SHP and KHP-III; the values increased proportionally with the increase in the concentration of KSF. This result was expected, due to the high ash content in the KSF (4.33%). López–Vargas et al. [20] also observed this fact in relation to hamburger with added passion fruit residue. In that study the control treatment had 1.94% and the treatments with 2.5 and 5% added residue showed values of 2.15 and 2 26%, respectively.
The lipid values ranged from 14.20 to 16.61%. The treatments differed (p<0.05) between each other; the highest values were found in the pâtés with the highest concentrations of KSF (1.0 and 2.0%). This can be explained by the lack of homogeneity of the raw material, since the meat was weighed and processed separately.
Regarding the content of dietary fiber, this ranged from 1.46 to 3.43%. There were significant differences between the treatments, and the content increased proportionally with the concentration of KSF. This result was expected considering that the KSF contained 28.79% of total dietary fiber. Brazilian legislation [19] defines a product as being rich in fiber when the minimum dietary fiber content is 6 g/100 g and any product that is a source of fiber should present a minimum content of 3 g/100 g. Thus, treatments KHP-II and KHP-III could be classified as foods that were sources of fiber because they contained 3.01 and 3.43 g of dietary fiber per 100 g of pâté. Values similar to those found in the present study were found by Preuss et al. [21] in pâtés where the values ranged from 1.0 to 3.5% of dietary fiber, when 3% rye fiber, passion fruit and pea were added.
Measurement of pH and water activity (aw)
The results of the effect of the KSF on the pH and water activity of the ham pâtés, stored at 4°C for 63 days, are shown in Figure 1.
Figure 1: pH values of ham pâtés with different added different concentrations of KSF during 63 days of storage at 4°C. SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control Ham Pâté, without antioxidant; KHP-I: Ham Pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II: Ham pâté with 1.0% KSF and KHP-III-Ham pâté with 2.0% KSF.
Significant difference in pH was observed between the treatments and also during the period of storage. On the first day the Standard Treatment (SHP) showed the lowest pH and the control treatment showed the highest pH. The treatments with added flour had intermediate pH values and even the KSF treatment had a pH level of 3.5. Knowing that the Control Treatment (CHP) contained no added sodium erythorbate or KSF, the addition of synthetic antioxidant contributed to the decrease in pH, which was also observed in a study by Perlo et al. [22], who found pH values of 6.6 in ham pâté with added sodium ascorbate, and 6.7 without the addition of antioxidant. After the twenty-second day of storage the pH decreased in all treatments, which can be explained by the activity of bacteria producing lactic acid, or compounds with low molecular weight formed from endogenous and exogenous activities in the product [23].
The water activity of the ham pâtés (Figure 2) ranged from 0.98 to 0.99. There was a significant difference between the treatments only on days 49, 56 and 63, when the SHP treatment had the lowest values. No difference was observed between the treatments by adding the flour, which had an AW level of 0.41. With regard to the storage period, there was a decrease. A similar result was observed by Amaral et al. [24] in lamb pâté, where the water activity reduced from 0.97 to 0.95 over 90 days. This decrease can be attributed to the decrease in pH values, because the water holding capacity of meat proteins is reduced when the pH approaches its isoelectric point (5.7), which accelerates dehydration and reduces water activity [10].
Figure 2: Water activity values (aw) of ham pâtés with different added concentrations of KSF during 63 days of storage at 4°C. SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control Ham Pâté, without antioxidant; KHP-I: Ham pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II: Ham pâté with 1.0% KSF and KHP-III: Ham pâté with 2.0% KSF.
Measurement of color
There was difference in color in all the treatments, as well as during the storage time. The L* variable varied from 70.78 to 60.60, decreasing with increasing concentrations of KSF. This was due to the presence of pigmented materials from the KSF or oxidative darkening of phytochemicals, which occurs at high temperatures [25].
On the first and seventh days of storage the L* values of SHP and CHP were similar, and had higher values, followed by KHP-I, KHP-II and KHP-III. On days 14 and 63, the SHP and CHP treatments differed significantly, probably due to the action of sodium erythorbate, which also aids in color formation [26]. On the last day of storage, treatment KHP-I had luminance values similar to SHP and CHP, which can be explained by the high humidity of KHP-I (63.98%). Typically meat products with higher humidity generate higher luminance values [25].
The a* values were different between treatments and during storage, except for KHP-III, where similar values were maintained during storage. The SHP and CHP treatments had the lowest values, which can be explained by the addition of sodium erythorbate in the standard treatment, which assists in the development of color because ascorbic acid donates electrons to the nitrite, leading to the formation of nitric oxide [27]. These findings in the present study are in agreement with a study by Perlo et al. [22], who found values of 6.22 in ham pâté without added antioxidant and 8.71 in ham pâté with added sodium ascorbate. Regarding the treatments with added KSF (KHP-I, KHPII and KHP-III), an increase was observed in the a* (red) value with increasing concentrations of KSF. The a* values in the present study decreased during the storage period in all the treatments, which can be attributed to the oxidative degradation of nitro-pigments, as well as the transformation of oxymyoglobin from a bright red color to brown metmyoglobin. Amaral et al. (2015) also observed a fall in the a* value from 18.56 to 16.54 in lamb pâté after 75 days.
Regarding the b* variable, SHP and CHP were similar, except for the first day, when the SHP treatment had a higher value. This may have been attributed to the higher fat content (15.25%), which may have interfered with the analysis. The averages of the b* values were similar to those reported by D’arrigo et al. [28] in pig liver pâté, which were 16.35.
Lipid oxidation–TBARS
Differences were observed in the TBARS values (Figure 3) between the treatments and during the 84 days of storage, except on day 56, when the results were similar. Unexpectedly, on the first day the standard pâté showed the highest values. This fact can be attributed to the sodium erythorbate, which is a compound used in meat products to enhance color and to inhibit microbiological growth and lipid oxidation. However, depending on the added concentration it can act as a pro-oxidant [27]. This result may also have been associated with the higher fat content in the SHP treatment (15.25%) (Table 2), given that the higher the amount of fat the greater the tendency of a product to oxidize, as observed by Estévez et al. [29], in pig liver pâté with different concentrations of fat.
Figure 3: Lipid oxidation of ham pâté with different added different concentrations of kiwi fruit skin flour during 84 days of storage at 4°C. SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control Ham Pâté, without antioxidant; KHP-I: Ham pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II: Ham pâté with 1.0% KSF and KHP-III: Ham pâté with 2.0% KSF.
The treatments with added KSF had lower results and in general they did not differ between themselves. This may have been related to the presence of fibers, which can be classified as antioxidant fibers, and which are associated with the polyphenols present in both the soluble fraction and the insoluble fraction [30]. Similar results were observed in a study by Mendes et al. [31]. In that study, fibers were added to byproducts of wine in salami, significantly reducing the development of lipid oxidation.
There was a decrease in the TBARS index during the first days of storage and a slight increase by the end of the storage period. The gradual reduction in TBARS values until the 49th day may have been associated with an increase in the concentration of highly polar products resulting from the polymerization of the products of secondary oxidation, which react with malondialdehyde, reducing the amount of the latter available to react with thiobarbituric acid [32]. The results of the present study are in accordance with a study by Yunes et al. [33], which found low TBARS values in bologna and a reduction of those values during storage. The standard bologna, which only contained pork fat, had values of 0.22 (day 0), 0.12 (day 30) and 0.14 (day 60) mg MDA/Kg. The authors attributed this fact to the decomposition of malondialdehyde by some types of microorganisms, or by the oxidation of malondialdehyde, which formed products that do not react with thiobarbituric acid.
The addition of ingredients, such as oregano, pepper blend and soy protein isolate, may have had a positive influence on the lipid stability because these ingredients possess antioxidant activity.
Previous studies of meat products have shown that TBARS values below 1.0 mg MDA/Kg of sample have no unpleasant odour and taste [34]. In the present study, no treatment reached this value; however, the treatments with added KSF had the lowest TBARS levels, indicating that the KSF acted as a natural antioxidant.
Level of vitamin C and antioxidant activity
All the treatments showed the presence of ascorbic acid (Figure 4a) due to the addition of 0.4% of 10% citric acid and 0.9% of spices. Treatment SHP showed the highest value of vitamin C, which was mainly attributed to the addition of 0.5% sodium erythorbate. The treatments KHP-I, KHP-II and KHP-III also showed higher values than CHP due to the addition of KSF. It was also observed that the vitamin C content decreased from day 7 to day 60 in all the treatments, except for KHP-I. This was due to the degradation of vitamin C in the presence of light and/or oxygen during the preparation of the analysis [35].
Figure 4: Vitamin C (4a) and antioxidant activity by DPPH method of pâtés with different added concentrations of KSF (4b). SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control Ham Pâté, without antioxidant; KHP-I: Ham pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II: Ham pâté with 1.0% KSF and KHP-III: Ham pâté with 2.0% KSF.
The antioxidant activity (Figure 4b) ranged from 47.56 to 96.15% of inhibition of the DPPH radical. There were differences (p<0.05) between the treatments and during storage. Treatment SHP showed the highest antioxidant activity, which was related to the addition of commercial synthetic antioxidant. The treatments KHP-I, KHP-II and KHP-III showed intermediate values; this was related to the addition of KSF, which presented antioxidant activity.
Microbiological stability
The National Health Surveillance Agency (ANVISA) Resolution No. 12 [15] establishes the technical regulation for microbiological standards for foods. According to that resolution, the ham pâtés analyzed in this study were within the standards required for meat products (semi-preserved products), where the maximum values allowed for coliforms at 45°C/g is 103 CFU/g for coagulase-positive Staphylococcus. For sulfite-reducing Clostridium the maximum permitted value at 46°C is 5 × 102 CFU/g, and for Salmonella sp the required limit is absence in 25 g.
The coliform count at 35°C for all the treatments was less than 101 CFU/g. These results can be attributed to the heat treatment (up to 73°C within the mass) to which the ham pâtés were submitted, given that the in natura KSF that was added showed a count of 5 × 103. However, in relation to coliforms at 45°C both the KSF and the pâtés had values below 101 CFU/g.
The total mesophilic counts for the KSF showed higher values than those found in the ham pâtés, which was related to the heat treatment to which the pâtés were submitted.
To verify the shelf life of the ham pâtés stored under refrigeration at 4°C the aerobic mesophilic and psychrotrophic counts were performed for 63 days and the results are shown in Table 3.
| Attributes | SHP | CHP | KHP-I | KHP-II | KHP-III |
|---|---|---|---|---|---|
| Color | 3.98ns ± 0.62 | 3.96ns ± 0.56 | 4.94ns ± 0.58 | 5.34* ± 0.59 | 6.22* ± 0.61 |
| Odor | 4.10ns ± 1.06 | 3.40ns ± 0.97 | 3.87ns ± 1.30 | 4.04ns ± 1.25 | 4.30ns ± 1.22 |
| Taste | 4.18ns ± 1.10 | 3.58ns ± 0.99 | 3.80ns ± 1.21 | 4.42ns ± 1.26 | 5.04ns ± 1.44 |
| Texture | 3.94ns ± 0.91 | 4.36ns ± 0.85 | 4.40ns ± 1.08 | 3.98ns ± 1.02 | 3.58ns ± 1.12 |
*Differs significantly from the standard sample. ns Does not differ significantly from the standard sample (Dunnett’s test)
Table 3: Multiple comparison tests for pâtés with different added concentrations of KSF.
From the count of aerobic mesophilic microorganisms it can be seen that there were significant differences between the treatments and during the storage period. The mesophilic counts in the pâtés with added KSF were similar to the standard and control. Regarding the storage period, the treatments showed a gradual increase in the count with the passage of time.
Regarding the psychotropic bacteria count, there was a significant difference between the treatments on days 42 and 63, and the count began to be significant after 42 days.
It can be seen that the treatments had counts of less than 106 CFU/g for both mesophilic and psychrotrophic microorganisms. According to Terra, counts up to 106 CFU/g are considered to be acceptable in meat products. These results prove the feasibility of storing the pâtés in a refrigerator (4°C) for at least 63 days, with no decrease in the microbiological and organoleptic quality. These results are in accordance with a study by Amaral et al., which also failed to find the development of microorganisms capable of compromising the quality of pâté for a period of 45 days.
Sensorial analysis
Fifty tasters were recruited for the hedonic scale acceptance test. Of these, 35 were women and 15 men; 38 were aged between 19 and 30; 7 were aged between 31 and 50; and 5 were older than 50. Fifty tasters were recruited for the multiple comparison tests. Of these, 39 were women and 11 men; 37 were aged between 19 and 30; 8 were aged between 31 and 50; and 5 were older than 50.
Multiple comparison tests: The discriminatory test shows no significant difference between the treatments by Dunnett’s test compared to the standard (value 4; equivalent to sample SHP) for most attributes, except for color, in the KHP-II and KHP-III samples, which received values near 5 (moderately darker than the standard) and 6 (much darker than the standard), respectively (Table 3).
SHP–Standard ham pâté, with sodium erythorbate; CHP-Control ham pâté, without antioxidant; KHP-I–Ham pâté with 0.5% kiwi fruit skin flour (KSF); KHP-II-Ham pâté with 1.0% KSF and KHP-III-Ham pâté with 2.0% KSF. Scores: 1: Extremely lighter, less firm or less intense than the standard. 2: Much lighter, less firm or less intense than the standard. 3: Moderately lighter, less firm or less intense than the standard. 4: The same as the standard. 5: Moderately darker, firmer or more intense than the standard. 6: Very much darker, stronger or more intense than the standard. 7: Extremely darker, firmer or more intense than the standard.
Regarding the attribute of odor, there was no statistical difference between the treatments, but the CHP and KHP-I samples received similar scores that were equivalent to a slightly less intense odour than the standard sample (mean<4), while the SHP, KHP-II and KHP-III samples were evaluated as having a slightly more intense odour than the standard (mean>4). In relation to the attribute of taste, none of the samples were statistically significantly different from the standard sample. The tasters reported the CHP and KHP-I samples as having a less intense flavour than the standard (mean<4) and for the SHP, KHP-II and KHP-III samples they reported a more intense flavour than the standard (average>4). In relation to the attribute of texture, once again none of the treatments differed from the standard. The SHP, KHP-II and KHP-III treatments were evaluated as being slightly less firm than the standard (mean<4), which contradicted the evaluations given to the SHP treatment as being the same as the standard, and treatments CHP and KHP-I as being slightly firmer than the standard.
Hedonic scale test, acceptability index and intention to purchase: In the hedonic scale, the scores awarded by the tasters for all the attributes were between 4 (‘neither liked nor disliked’) and 6 (‘liked a lot’) in the seven-point structured scale (Table 4).
| Attributes | SHP | CHP | KHP-I | KHP-II | KHP-III |
|---|---|---|---|---|---|
| Color | 5.56a ± 0.92 | 5.50a ± 1.09 | 5.30a ± 1.03 | 5.36a ± 0.87 | 5.26a ± 0.98 |
| Odor | 4.82b ± 1.30 | 5.28ab ± 1.07 | 5.34ab ± 1.06 | 5.56a ± 0.95 | 5.02ab ± 1.16 |
| Taste | 4.56b ± 1.37 | 5.06ab ± 1.13 | 5.04ab ± 1.12 | 5.26a ± 1.17 | 4.80ab ± 1.24 |
| Texture | 5.08a ± 1.13 | 5.18a ± 1.10 | 5.14a ± 1.03 | 4.98a ± 1.26 | 5.16a ± 0.99 |
| Appearance | 5.28a ± 1.08 | 5.36a ± 0.99 | 5.34a ± 0.91 | 5.48a ± 0.78 | 5.24a ± 0.93 |
Values presented as mean ± standard deviation. a-cDifferent letters on the same line indicate significant difference (p<0.05) by Tukey’s test
Table 4: Hedonic scale test for pâtés with different added concentrations of KSF.
SHP–Standard ham pâté, with sodium erythorbate; CHP-Control ham pâté, without antioxidant; KHP-I–Ham pâté with 0.5% kiwi fruit skin flour (KSF); KHP-II-Ham pâté with 1.0% KSF and KHP-III-Ham pâté with 2.0% KSF. Scores: 1: Disliked very much; 2: Disliked a lot; 3: Moderately disliked; 4: Neither liked nor disliked; 5: Liked moderately; 6: Liked a lot; 7: Liked very much.
The attribute of color did not differ significantly between the treatments; however, it was clear that the samples with added KSF had lower acceptance values. The means ranged between ‘liked moderately’ and ‘liked very much’. With regard to the attributes of odor and flavour, the means were different; the KHP-II sample received higher scores (between ‘liked moderately’ and ‘liked a lot’) while the SHP sample received lower scores (between ‘neither liked nor disliked’ and ‘liked moderately’). These data show that the addition of KSF can positively enhance the odor and flavor of ham pâté. The attributes of texture and overall appearance showed no significant difference between the means, which generally ranged from ‘liked moderately’ to ‘liked very much’, except for the KHP-II sample, which received a score lower than 5. Barros et al. also reported better acceptance for hamburgers with 30% added cashew fiber than the standard hamburger. The latter study also applied a multiple comparison (acceptance) test to compare hamburgers with added cashew fiber to a standard hamburger and found that the formulation with 30% added cashew fiber was better accepted than the standard according to 43.4% of the testers. The intention to purchase test (Figure 3) showed that 78% of testers would buy the SHP formulation; 82% would buy CHP; 82% would buy KHP-I, 76% would buy KHP-II and 36% would buy KHP-III (Figures 5 and 6).
Figure 5: Microbiological stability of pâtés with different added concentrations of KSF during 63 days storage at 4°C. SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control Ham Pâté, without antioxidant; KHP-I: Ham pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II: Ham pâté with 1.0% KSF and KHP-III: Ham pâté with 2.0% KSF.
Figure 6: Intention to purchase test for pâtés with different added concentrations of KSF. SHP: Standard Ham Pâté, with sodium erythorbate; CHP: Control Ham Pâté, without antioxidant; KHP-I: Ham pâté with 0.5% Kiwi Fruit Skin Flour (KSF); KHP-II: Ham pâté with 1.0% KSF and KHP-III: Ham pâté with 2.0% KSF.
The kiwi fruit skin flour (KSF) acted as a natural antioxidant and it can aid in reducing lipid oxidation in meat products.
The addition of KSF resulted in an increase in the dietary fiber content of the ham pâtés, and the KHP-II and KHP-III treatments could be considered as a source of fiber. The physicochemical characteristics were only slightly affected by the addition of KSF; there was a darkening in color in the pâtés in conjunction with increased concentrations of KSF. The microbiological stability was unchanged. Regarding sensory acceptance, the multiple comparison tests showed that the formulations with added KSF did not differ from the standard, except for the attribute of color. In the formulations with higher concentrations of KSF (1.0 and 2.0%) the hedonic scale test indicated that the samples were well accepted, except the pâté with 2.0% KSF. The purchase intent test highlighted the fact that only the sample with the highest concentration of KSF would not be purchased by the testers. The use of KSF at a concentration of 1.0% proved to be feasible as an ingredient in meat products in terms of acting as a natural antioxidant and a source of dietary fiber without changing the quality of the ham pâtés.
The authors wish to thank the Coordination of Improvement of Higher Education Personnel (CAPES) for providing an MA scholarship to the first named author and also the Department of Food Science and Technology at the Federal University of Santa Maria, Brazil.