Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Paper - (2020)Volume 10, Issue 1
Background: Date palm is considered as one of the essential fruits across various Arab countries, particularly in Saudi Arabia. The physical and chemical composition of the date fruits in different cultivars has been reported in many countries. The study aimed to assess the correlation between amylase activity & reducing sugar content in date fruits. It also aims to analyze the increase in amylase activity with the proportional increase in the reducing-sugar content.
Methods: Qualitative analysis was used to evaluate the correlation between amylase activity & reducing sugar content in date fruits. Biochemical changes associated with the ripening of four different varieties of date fruits: sukkari, hilwa, sullaj, and khalas were examined.
Results: The analysis showed that ripening of date fruits increased sugar content and enzyme activities. Correlation between amylase activity & reducing sugar content in date fruits was initially observed in Khalas and then in other varieties of date fruit. The ripening of all fruits was consistent in all date fruits varieties examined through the analysis exhibited considerable variations. The induction of amylase activity showed chronological and quantitative variations among date varieties.
Conclusion: It suggests that biochemical attributes of the date palm must be considered for its expanded cultivation.
Amylase activity; Date fruit; Hilwa; Khalas; Reducing sugar; Sukkari
Date Palm (named as Phoenix dactylifera) belongs to Arecaceae, a palm family [1]. It is a staple food item and is cultivated across the Middle East and North Africa region [2]. Saudi Arabia is the second-largest date-producing country, which harvests more than three hundred types of dates, each with its texture, size, and taste [3]. The date fruit in Saudi Arabia is the most grown fruit with the annual production of 1.08 million tons of date from four hundred different cultivars and twenty-four million date palm trees. The production represents about 13.67% of the total world production of 7.9 million tons of dates. It is a fact that amylase and sugars are two major constituents of the date palm, which are directly associated with the process of maturation through pollination. Generally, the consumption of date fruits occurs at three stages, where the first is fresh (i.e., Khalal stage), second is crisp to succulent (i.e., Rutab stage), and last is soft pliable (i.e., Tamr stage).
The physical and chemical composition of the date fruits in different cultivars has been reported in many countries [4]. Dates contain a small amount of B1 thiamine, B2 riboflavin, nicotinic acid, and vitamin C [2]. Lima, Chitarra, and Chitarra indicate that the metabolic differences in fruits result due to climate change [5]. Despite it, the information concerning the enzyme activity in the starch hydrolysis is limited for increasing the fruit growth before and after the maturity. Similarly, amylase is an effective enzymatic constituent, which breaks the bond between the carbohydrates leading to polysaccharides and hydrolysis reactions [6]. Amylase is synthesized in many plants and fruits during ripening and causes them to become sweeter [7].
This enzyme is mainly found in plants, bacteria, and animals. Alpha-amylase is an important enzyme in the metabolic and digestive processes. Amylase can be categorized into three types that include the beta-amylase, alpha-amylase, and gamma-amylase [8]. It hydrolyzes the polysaccharide bonds in all three types of amylase. Amylase is typical in the digestion of starch into sugar to make it an accessible source of energy for the body. It is also present in the blood that helps to digest white blood cells. Dates due to its phenolic contents and high fiber can play an important role in the deterrence of modulation of the immune and cardiovascular disease.
The amylase belonging to the heterogeneous group of enzymes catalyzes the hydrolysis of α-1, 4-glycosidic bonds of polysaccharides to yield dextrin, oligosaccharides, maltose, and D-glucose. A group of enzymes is known to attack insoluble starch and starch granules, held in aqueous suspension. The products of amylase action constitute a major source of energy in the diet [9]. This fact has generated considerable interest in this class of enzymes, and as such, amylases are important enzymes for many diverse industries, including food processing, pharmaceutical, and textile industries.
Glucose, fructose, and sucrose have been identified as core sugar elements, which are commonly found in date fruits. The richness of the varieties presents in reducing sugar suggested that the presence of pronounced invertase activity is considerably lower than the content of sucrose. Despite the rising awareness of the particular carbohydrate role as food for the human body, many complications in the field are away from being solved owing to the structural and chemical complexity of the sugars [10].
Even though various studies have been conducted on date fruit, very few have considered the amylase activity and the sugar profile of the date fruit content during ripening [11-13]. The free pentose and hexose content of many foods are commonly reported as the total reducing sugars. The non-reducing sugar has been usually reported due to the oligosaccharide content of food calculated to be sucrose. With the steady development of the role of individualspecific sugars in the processes of metabolism, it is becoming increasingly essential that the specific sugars in foods be recognized in the concentration tabulated. The effects of increased amylase activity with the proportional increase in the reducing-sugar content of fruits have been analyzed by examining the correlation between reducing sugar content and the amylase activity in date fruits. This study examined the biochemical changes associated with the ripening of four different varieties of date fruits: sukkari, hilwa, sullaj, and khalas. It is significant as it supplies incremental information concerning the ripening process of date fruits for the sugar and enzyme activity. Some considerable variations have been observed during the ripening process, and it has assisted the Food system and agriculture. There is a need for an adequate and more productive pattern to adapt to the adverse effects of the change in the climate.
Methods
Date fruits were collected at various stages of ripening for four different date palm varieties (Sukkari, Hilwa, Sullaj, and Khalas) [14]. These were collected from a private farm in Riyadh, Saudi Arabia. Ten different sets of samples representing ten different stages of maturity were collected where each date fruit variety was examined.
Materials
For the study, different materials were gathered, including Starch, which was acquired from the Sigma Chemical Company (USA). From the Winland, 3,5,-Dinitrosalicylic acid, Carboxymethyl cellulose, sodium chloride, Coomassie brilliant blue G-250, and catechol were collected. The polygalacturonic acid was collected from Fluka AG, Chem. Potassium-sodium (+)-tartrate, sulfuric acid, methanol, anthrone, and glucose were gathered from the BDH Chemicals (England). From Riedel-de Haen (Germany), sodium acetate was purchased. Other chemicals were collected from the local sellers, ensuring their highest purity.
Sample preparation and extraction
The date samples were thoroughly rinsed using distilled water and blotted dry. The sample was categorized into two parts, buffer comprising of 50 mM sodium acetate (pH 5.0), as well as NaCl 100 mM, which was used for determining the sample protei content, reducing sugar, total sugars as well as different activities of the enzymes. Methanol was used for the second sample to determine its pigment content.
Extraction with aqueous buffer
Following pit removal, the sample weighs determined. These were then chopped into small pieces followed by their maceration in the ice-cold extraction before with the use of mortar and pestle. For 15 minutes, the centrifugation of the homogenate occurred at 15,000xg in a Beckman J-20 centrifuge at 4°C. For all samples, the extraction of one gram occurred using a 3 mL buffer.
Extraction with methanol
Following pit removal, the sample weight was determined. The sample was then chopped into small pieces followed by their maceration in methanol through mortar and pestle. It also included the methanol ratio of 5 mL on one gram of date. Pigments were determined using supernatant.
Determination of sugar content
For determining the total sugar content, Van Handel defined anthrone agent was used. Since most of the extracts had increased sugar content, all the extracts were diluted (1-100) before the determination of the sugar. Glucose was used as a reference for calculating the exact sugar content. While reducing sugar content is determined using dinitrosalicylic acid (DNS). The method tested the presence of free carbonyl group in sugars (C=O, or the so-called reducing sugars). The reaction involves the oxidation of the aldehyde functional group present in sugars with simultaneous reduction of 3, 5- dinitrosalicylic acid (DNS) to 3-amino, and 5-nitrosalicylic acid under alkaline conditions. In assays, 1 mL sample was mixed with 1 mL DNS reagent, and then the mixture was placed in a boiling water bath for 5 minutes to conduct the assays. 3 mL of water was added to the mixture after it was cooled down, then the absorption level was measured at 540 nm against a blank prepared using 1 mL distilled water and 1 mL of DNS reagent.
A standard glucose curve was used for determining the concentration of reducing sugars, except that 1 mL samples containing 0, 0.2, 0.5, 1.0, 2.0, 3.0, or 5.0 mM glucose. Under these conditions, the absorbance at 540 nm was linear with glucose concentration in the range 0-5 mM. Assays were conducted by mixing 1 mL sample with 1 mL DNS reagent, then placing the mixture in a boiling water bath for 5 minutes. After the mixture had cooled down to room temperature, 3 mL water was added, then the absorption was measured at 540 nm against a blank prepared using 1 mL distilled water and 1 mL of DNS reagent. The reducing sugar content of the extract was reported as equivalent glucose concentration.
Amylase, Pectinase and Cellulase assays
The catalyzing of the reactions occurred for amylase, pectinase, and cellulase, where conversion of polysaccharides occurs for monoand oligosaccharides. Enzymes' all activities are measured through the sugar content release reduction in the reaction.
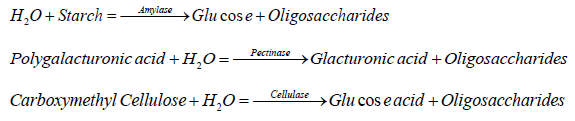
The primary problem observed high reducing sugar content is some of the data extracts, where the extracts with high sugar content are more likely to provide improved background reading, which may result in enzyme inhibition. Thereby, gel filtration chromatography of the date extract occurs for protein separation from the soluble sugar. In Sephadex G-25 columns (1x10 cm), the application of date extracts occurs, which were previously equilibrated with 50 mM sodium acetate buffer (pH 5.0). The same buffer was used for eluting the columns along with the pooling of fractions, which comprised of protein and were used for enzyme activity and protein content determination.
Determination of amylase
The amylase activities were determined in parallel assays simultaneously. In each case, the assay mixture (1 mL total volume) contains 200 μl desalted extract, 450 μl appropriate substrates, plus 350 μl sodium acetate buffer (50 mM, pH 5.0). The substrates were 0.5% (w/v) of either starch for amylase activity, polygalacturonic acid for pectinase activity, or carboxymethyl cellulose for cellulase activity. Before the determination of the released reducing sugar, the mixtures were incubated for 24 hours at room temperature. Amylase catalyzed similar reactions, in which polysaccharides were converted into mono- and oligosaccharides. Thus, the enzymes activities can be assessed by measuring the amount of reducing sugar level released during the reaction.
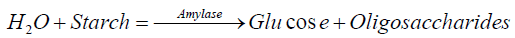
The date fruit ripening process has been examined using four different varieties of dates. In all varieties examined, the ripening of date fruits resulted in increased protein content, sugar content, certain enzyme activities, and synthesis of certain polyphenols.
Amylase and sugar activity in Sukkari
The analysis of sugar and amylase activities shows considerable changes in sukkari fruit sugar content during the various stages of ripening. The lowest total-sugar content (62 mg/g date or 6% by weight) was observed at the early stages of fruit ripening. The highest total-sugar content (873 mg/g date or 87% by weight) was recorded in the Tamr stage. In principle, the determination of total sugar implies a determination of all saccharides (poly-, di-, and monosaccharides). The determination of total sugar probably includes di- and monosaccharides only; polysaccharides would have precipitated out under the conditions of extraction.
Changes in total sugars and/or reducing sugars might have been the result of increased activity of enzymes responsible for starch hydrolysis into simple sugars. Results have shown that the transient increase in the activity of amylases was concurrent with the increase in reducing sugars content of sukkari. Figure 1 contrasts the changes in amylase activity versus reducing sugars during the various stages of sukkari ripening [15].
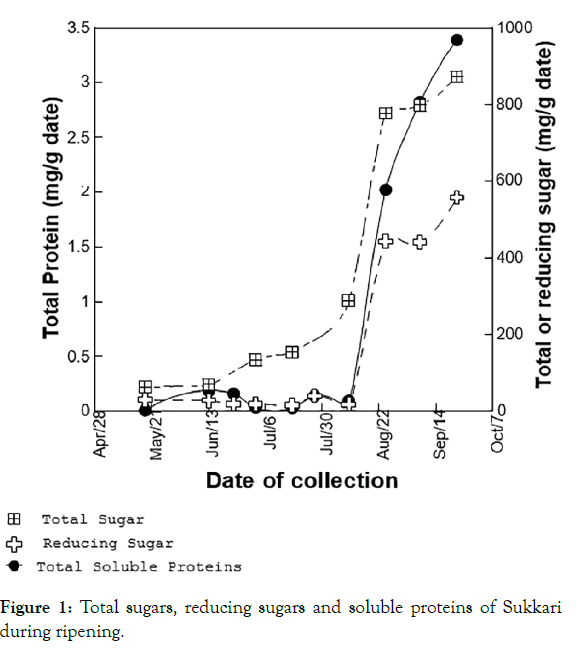
Figure 1: Total sugars, reducing sugars and soluble proteins of Sukkari during ripening.
Amylase and sugar activity in Hilwa
The sugar content represented differential stages of hilwa fruit ripening by two methods. The first method showed the reducing and non-reducing sugars measurement of total sugar content. However, the second method showed only reduced sugar measurement. The results revealed that hilwa fruit had efficient levels of sugar at the initial ripening stages (73 mg/g date or about 7% by weight for the period of June through July 18). The sugar content increased dramatically during the next stages of ripening. The highest total sugar content was 782 mg/g date or about 78% of date fruit by weight was recorded on August 25th, at which hilwa fruit was at the tamr stage. A study conducted by Al-Hooti et al. [16] suggested that among several different procedures applied to the production of date syrup, the use of cellulose and pectinase enzymes provided the highest recovery of the total diluted values and also the concentrated date syrup of variety of dates, indicating light color. The findings of this study have suggested that the probability of employing cellulose and pectinase enzymes to yield concentrated syrup from the variety of tamr fruits is for utilizing it in product development.
The initial stage of the ripening process of the total sugar content of hilwa was low. It was evenly distributed between non-reducing and reducing sugar. The results have indicated that most of the increases in the sugar content of hilwa fruit were not clear in the initial stages; but, the later stages of ripening showed the increase in sugar content that was due to the increase in reducing sugar content (Figure 2). The non-reducing sugar content was accounted for less than 12 percent of the total sugar.
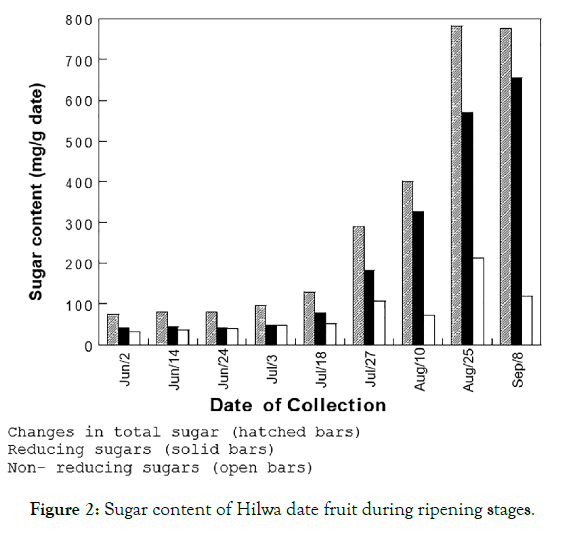
Figure 2: Sugar content of Hilwa date fruit during ripening stages.
In hilwa, the induction of amylase activity was more pronounced than that of sukkari. The level of amylase activity remained lower in both varieties and categories of date fruit. Further ripening of the date fruit with the decline in amylase activity can be attributed to many different factors that include the transient expression, protein degradation, or senescence of fruit, as evidenced by their low moisture content. The process of ripening increased the soluble protein, reducing sugars and the total sugar. Ripening increased the total sugars, reducing sugar and soluble protein. Furthermore, changes in total sugars appeared to mirror those of reducing sugars (most probably glucose and fructose).
This aspect suggested that enzymes, which have increased the reducing sugars of hilwa, were induced before the events that led to major changes in protein content. The change in color of hilwa also occurred during date ripening (from green to red to dark brown) might reflect changes in the activity of several enzymes. The reducing sugars content of sullaj also changed drastically during ripening. The lowest reducing sugar content (10 mg/g date or 1% by weight) was observed at the early stages of fruit ripening (samples collected in May), and the highest (564 mg/g date or 56% by weight) was recorded for samples collected on August 25th, when the fruit was at the Tamr stage. In general, amylase activity present in the extract of sullaj can be considered low during all stages of ripening (<0.31 mM glucose produced per hour per mL extract).
Amylase and sugar activity in Sullaj
The analysis of sugar activity, along with the amylase activity, has shown that sullaj fruit underwent some prominent changes in the sugar content during many stages of the ripening process. The total sugars content of sullaj increased from 4% (by weight) during the early stage of fruit ripening (May 6) to about 76% for samples collected on August 10, before declining to about 70% at the final stage of maturation. The reducing sugars content of sullaj has also been observed to be changed drastically during ripening (Figure 3). The lowest reducing sugar content (10 mg/g date or 1% by weight) was observed at the early stages of fruit ripening (samples collected in May), and the highest (564 mg/g date or 56% by weight) was recorded for samples collected on August 25, when the fruit was at the tamr stage.
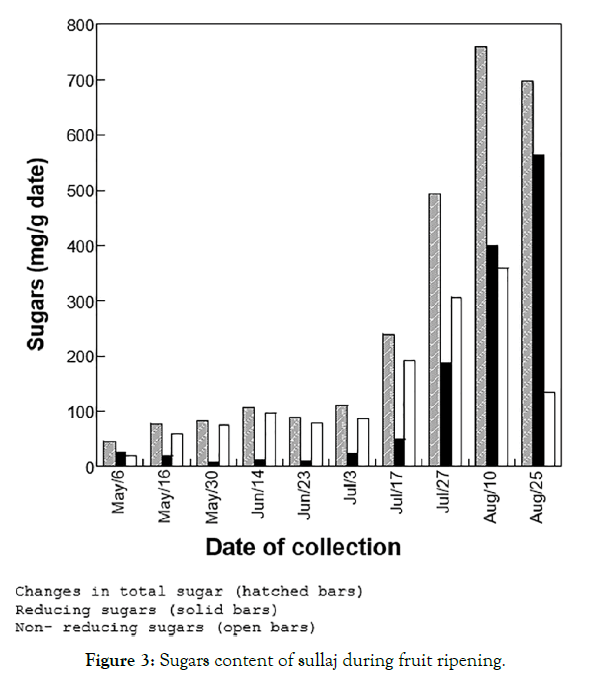
Figure 3: Sugars content of sullaj during fruit ripening.
The change in levels of amylase activity during the ripening of sullaj was examined. In general, amylase activity present in the extract of sullaj can be considered low during all stages of ripening (<0.31 mM glucose produced per hour per mL extract). The results have observed changes in amylase activity versus reducing sugars during the various stages of sullaj ripening.
Amylase and sugar activity in Khalas
The results have revealed that the khalas fruit experienced substantial changes in their reducing and/or total sugar content during the various stages of ripening. At the early stages of ripening, this date variety had a low sugar content of 53 mg/g date or 5% by weight, total, or reducing sugars. At the later stages of ripening, the sugar content of khalas increased dramatically and reached a maximum of 745 mg/g date or 74% by weight at the final stage of maturation. The vast majority of sugars in khalas were reducing sugars, with only small amounts of non-reducing sugars throughout the ripening process.
Extracobserved while other categories showed some consistent values. The amylase activity substantially impacts the adapting and adjusting of the date fruit sugar content. Khalas was the first date that showed amylase activity in the mid of July chronologically, followed by sullaj, hilwa and sukkari in early September. The amylase induction was longer than that of the bulk protein. The highest level of amylase protein was showed by khalas; whereas, the sukkari had the lowest level (Figure 4). The increase in amylase activity appeared to coincide with increased reducing sugar content of date fruits, suggesting that starch hydrolysis contributes to the production of reducing sugars in all date varieties.
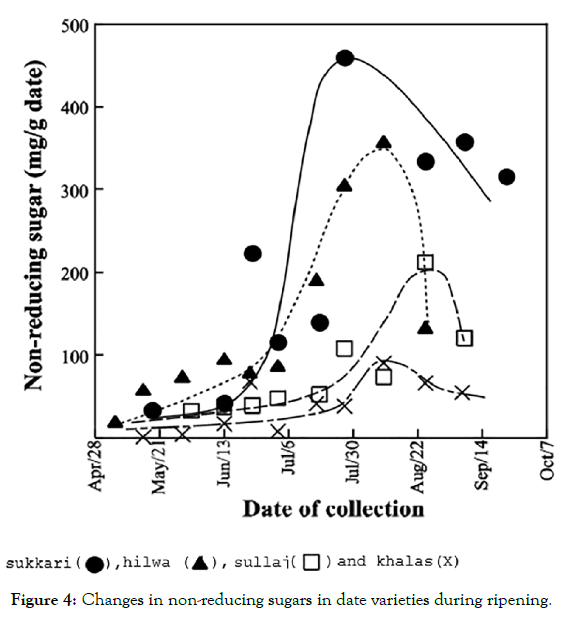
Figure 4: Changes in non-reducing sugars in date varieties during ripening.
The increase in the amylase activity increased to clash with the increased content of sugar of the date fruits [17,18]. It suggested that starch hydrolysis might contribute to reducing sugar production in all types of dates. The variation was present throughout the ripening process of the date fruit production. Other hydrolyzing enzymes were also certainly involved in the production of reducing sugar. The results obtained in the study are in line with the previous study of [19,20]. It also showed that the variation in the sugar content might be due to its involvement in other components of content. Boss, Böttcher, and Davies [21] suggested use of other components in the date fruit to enhance sugar content profile in date fruits. El-Sohaimy and Hafez indicated that the ripe date sugar content is about 80 percent, while the remaining 20 percent contains fat, proteins, as well as minerals comprising of sulfur, magnesium, copper, iron and fluoric acid [22]. Concerning climate change, Parajuli [23] highlights that content also varies based on exposure to a different climate. It exemplifies that exposure to direct sunlight leads to higher sugar content in the fruits as compared to the ones’ that are placed in a more shaded area.
The limitation of the study includes its collection of samples from a single farm in Saudi Arabia in a single region. A major problem encountered in measuring the amount of reducing sugars was that certain date fruits contained a high amount of reducing sugar when released during the conversion process of starch into glucose and oligosaccharides. As high sugar content will produce high background readings, so it may cause reaction inhibition of the involved enzymes. Therefore, extracts were exposed to gel-filtration chromatography to divide proteins from soluble sugars. Extracts were applied on Sephadex G-25 columns (1x10 cm), previously equilibrated with 50 mM sodium acetate buffer (pH 5.0). Columns were removed by using the solvent with the same buffer, and fractions containing protein were also pooled and utilized for the protein content and enzyme activity determination. The study also suggests that future researches should perform experiments for reducing the amalyze activity through genetic engineering. These researches can also administer a drug to the plants and then measuring amalyze/sugar content, which are likely to expand the research scope.
The results showed a significant correlation between amylase activity and the reducing agent in date fruits. Khalas has been observed with the highest level of amylase activity and the highest content of reducing sugar as well. Hilwa and Sullaj have also shown the intermediate level of reducing sugar and amylase activity. The level of amylase activity was compatible with reducing-sugars content in each fruit variety studied. The main fascinating aspect is that the date varieties examined in the study have different quality and taste for the people who consume it. According to the results, the sweetness of the date fruits might involve other components in addition to the context of sugars. It has been observed that all the varieties of the dates mentioned have the sugar content of about eighty to ninety percent by weight, most of which was in the form of reducing sugars. Different forms of sugar have different levels of glucose and fructose that are the common reducing sugar in date fruit. The combination of the two sugars is expected to be as sweet as sucrose and another major sugar component in date fruits.
The author is very thankful to all the associated personnel in any reference that contributed in/for the purpose of this research.
Citation: Al-Qarni SSM (2020) Correlation between Amylase Activity & Reducing Sugar Content in Date Fruits: A Case of Increased Amylase Activity with Proportional Increase in Reducing-Sugar Content of Fruits. J Nutr Food Sci 9:767. doi: 10.35248/2155-9600.20.10.767
Received: 30-Nov-2019 Accepted: 20-Jan-2020 Published: 27-Jan-2020 , DOI: 10.35248/2155-9600.20.10.1000767
Copyright: © 2020 Al-Qarni SSM. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.