Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2017) Volume 7, Issue 2
The potential uses of the starchy roots as a food and as an income-generating product in the rural areas could not be satisfactorily done in the developing countries especially in Ethiopia. Therefore Promoting and supporting the use of taro and yam can make a major contribution to the food security of Ethiopia and of the world as well. The present study focused on, the quantitative determination of antinutrient contents of the taro and yam samples cultivated in southwestern Ethiopia (Keffa zone, Benchmaji zone and Sheka zone). The parameters investigated were antinutrient factors such as: oxalate, Phytate and tannin. Antinutrient factors were determined by different standardized analytical methods and the results of both taro and yam samples were compared and analyzed accordingly. The result indicated that, the antinutrient levels of both raw taro and yam samples in this study were: Oxalate (0.062-0.085, 0.054-0.063 mg/100 g), Phytate (31.17-161.13, 55.72-179.74 mg/100 g) and tannin (4.18-6.72, 3.06-4.54 mg/100 g), respectively. The raw taro and yam tubers analyzed in this study were very low compared to the recommendations for patients with calcium oxalate kidney stones. In this study, in general, antinutritional contents of the current study had no significant health hazard even at raw level in comparison to their critical toxicity effect.
Keywords: Taro tubers; Yam; Oxalate; Phytate; Tannin
Root crops play a major role in the food security of many developing countries, and through the development and promotion of better management of these crops, the livelihoods of the poor people who depend on them will be improved. Historically, very little attention has been paid to root crops by policy-makers and researchers as most of their efforts have been concentrated on cash crops or the more familiar grains. Root crops were regarded as food mainly for the poor, and have played a very minor role in international trade. This misconception has lingered for so long because of the lack of appreciation of the number of people who depend on these root crops, and the number of lives that have been saved during famine or disasters by root crops. Root crops contain an appreciable amount of carbohydrate, vitamins and minerals and may have a competitive production advantage in terms of energy yield per hectare over cereals produced in ecologically difficult conditions [1,2]. The nutritional value is the main concern when a crop is being considered as a food source. Due to the emphasis placed on the nutritional value of food by consumers, a great need exists for information on the nutritional contents of root crops. The high starch content of most root crops is considered an excellent energy source [3]. It is also a tuber that is very rich in carbohydrates, ranging between 73-80% which is mainly starch at 77.9% and 1.4% crude fiber on Dry Matter (DM) basis [4]. In Ethiopia, reliable information on production of taro is hardly known and is most neglected food but the crop is highly grown by rural households in different areas of the country for special purposes after the 1984 famine [5,6]. In Ethiopia, taro is mainly prepared by boiling then the water drained off and served as a snack.
Tubers of domesticated yam are an important source of carbohydrate for millions of people in the tropics and sub-tropical regions of the world particularly in Africa, the Caribbean, parts of Asia, South and Central America and the Pacific. Some species of yam are of medicinal and ornamental value [7,8]. Yams are an important staple food and source of carbohydrate throughout West Africa. Also, they are important medicinally and have ritual and sociocultural significance [9]. A versatile vegetable, yams can be boiled, roasted, grilled or fried and served sliced, as balls, mashed, chipped and flaked. Fresh tubers can be peeled, chipped, dried and milled into flour. People consume yams, sweet in flavour, as a cooked vegetable fried or roasted. Presently, whole roasted yam has become a popular street or fast food in urban areas throughout the West African yam belt [10].
Compounds, which act to reduce nutrient utilization and/or food intake, are often referred to as antinutritional factors. These toxic compounds may occur in all parts of the plant, but the seed is normally the most concentrated source. Food crops regularly eaten have many beneficial nutrients but there are traces of antinutritional components such as cyanoglucosides, oxalates, phytic acid, phenolics, protease inhibitors, heavy metal etc. These antinutritional factors when consumed in foods may have adverse effects on health through inhibition of protein digestion, growth, and Fe and Zn absorption [11]. Like most foods of plant origin, taro contains a variety of anti-nutritional and toxic components such as oxalates, phytates, trypsin and amylase inhibitors, tannins and cyanide. Therefore, it is advisable to process taro before consumption [12-14].
Boiling is effective method in reducing water soluble antinutrients. For example boiling of root crops such as taro and cassava could lead to significant reduction of oxalates and cyanide respectively. Boiling also found to decrease some amount of soluble phytate [15]. Both taro and yam in their raw forms are toxic. The toxin is however destroyed by processing techniques such as cooking, soaking, ensiling and drying [14].
Although taro and yam are widely growing in Ethiopia particularly in its southern parts, they are underutilized crop and little is known about the antinutritional factors. In this study the antinutritional factors were determined using standardized analytical methods.
Statement of the problem
Even though there is a little bit practice of producing and eating the tubers of taro and yam particularly in the southern part of Ethiopia, the knowledge of the people to get good nutritionally reached food, and the proper conditions to be maintained is very limited. It has been observed that the potential uses of the starchy roots as a food and as an income-generating product in the rural areas could not be satisfactorily done in the developing countries especially in Ethiopia. Therefore Promoting and supporting the use of taro and yam can make a major contribution to the food security of Ethiopia and of the world as well.
General objectives
This research was designed to study the antinutrient levels of tubers of Colocasia esculenta L. (Taro, Godere) and Dioscorea alata (Yam).
Specific objectives
The specific objectives of this research were:
To investigate the antinutritional values of tubers of C. esculenta L. (Taro, Godere) and D. alata (Yam).
To compare the results obtained in the antinutrient levels of tubers of Colocasia esculenta L. (Taro, Godere) with the results in D. alata (Yam).
Sample collection and pretreatment
Samples of tubers of taro and yam were collected from south western of Ethiopia (Sheka, Keffa and Benchmaji zones). These areas were selected to represent the area that taro and yam is dominantly produced and consumed in the country. Both taro and yam samples were collected from three similar sites for the sake of comparison. The taro and yam samples selected contained large, middle and small tuber sizes that were not damaged during harvest and which were not attacked by pests. Three different Woreda was selected in each zone and samples were purchased from farmers in three sites in each Woreda and the collected samples were homogenized to represent the bulk sample. Then the collected samples were packaged in to polyethylene plastic bag, labeled and transported to laboratory for further treatment.
Sample preparation for both taro and yam samples
Both taro and yam samples were washed and peeled carefully using stainless steel knives and the peeled taro and yam samples were washed, rinsed with deionized water and then sliced. The slices were dried for 6 h in a hot air oven at 105. The dried taro and yam chips were powdered with a mortar and pestle with sieve size of 0.425 mm and packed in polyethylene plastic bags until analysis.
Anti-nutritional factor analyses
Determination of phytates
The Phytate content was determined according to method described by Latta and Eskin [16], and later modified by Vaintraub and Lapteva [17]. 0.5 g of dried sample was extracted with 10 mL 2.4% HCl for 1 h at ambient temperature and centrifuged (3000 rpm) for 30 min. The clear supernatant was used for the Phytate estimation.
One milliliter of Wade reagent (0.03% solution of FeC13.6H2O containing 0.3% sulfosalicylic acid in water) was added to 3 mL of the sample solution and the mixtures were centrifuged. The absorbance at 500 nm was measured using UV-VIS spectrophotometer. The Phytate concentration was calculated from the difference between the absorbance of the control (3 mL of water+1 mL Wade reagent) and that of assayed sample. The concentration of Phytate was calculated using phytic acid standard curve and results were expressed as of phytic acids in mg per 100 g dry weight. To prepare the phytic acid standard curve, a series of standard solution were prepared containing 5–40 mg/ml phytic acid in water [16]. Three milliliters of the standards was pipetted into 15 mL centrifuge tubes with 3 mL of water used as a zero level. To each tube 1 mL was added of the wade reagent, and the solution was mixed using a vortex mixer for 5 s. The mixture was centrifuged for 10 min and the supernatant read at 500 nm by using water as a blank. By Plotting the calibration curve (absorbance vs. concentration) and one can find out the slope and intercept. The Phytate content in taro were calculated using the following relation:
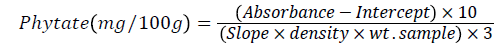
Determination of oxalate
Oxalate analysis
The oxalate contents of both raw taro and yam tubers were determined using the method developed by Iwuoha and Kalu [18]. This method involves the following three steps: digestion, oxalate precipitation and permanganate titration.
Digestion
At this step about 4 g of taro and yam flour was suspended in 190 ml of distilled water Contained in 250 ml conical (Erlenmeyer) flask; 10 ml of 6 M HCl was added and the suspension was then digested at 100 for 2 h, this was followed by cooling, and then solution was made up to 250 mL mark using distilled water and filtered in to another flask.
Oxalate precipitation
Duplicate portions of 125 ml of the filtrate were measured into a beaker and four drops of methyl red indicator was added, followed by the addition of concentrated NH4OH solution (drop wise) until the test solution changed from its salmon pink color to a faint yellow color (pH 4-4.5). Each portion was then heated to 90, cooled and filtered to remove precipitate containing ferrous ion. The filtrate was again heated to 90°C and 10 ml of 5% CaCl2 solution was added while being stirred constantly.
After heating, it was cooled and left overnight at 5°C. The solution was then centrifuged at a speed of 2500 rev/min for 5 min. The supernatant was decanted and the precipitate completely dissolved in 10 ml of 20% (v/v) H2SO4 solution.
Permanganate titration
At this point, the total filtrate resulting from digestion of 4 g of flour was made up to 300 ml. Aliquots of 125 ml of the filtrate were heated until near-boiling, and then titrated against 0.079 M standardized KMnO4 solutions to a faint pink color which persisted for 30 s. The calcium oxalate content was calculated using the formula:
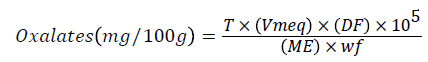
where T is the titre of KMnO4 (mg/100 g), (ml), Vmeq is the volume-mass equivalent in which 1 cm3 of 0.079 M KMnO4 solution is equivalent to 0.00225 g anhydrous oxalic acid), DF is the dilution factor VTA (where V is the total volume of filtrate (300 ml) and A is the aliquot used (125 ml), ME is the molar equivalent of KMnO4 in oxalate (KMnO4 redox reaction and wf is the mass of flour used.
Determination of tannins
Tannins contents of both taro and yam samples were determined according to the method developed by Burns [19]. About 1 g mass of dried sample was weighed in a screw capped test tube and 10 ml of 1% HCl in methanol was added to each test tube containing the samples. The test tubes were shaking for 24 h at room temperature using mechanical shaker and the tubes were centrifuged for 5 min. 1 ml of the clear supernatant was taken and mixed with 5 ml of vanillin-HCl reagent in another test tube and then the mixture was allowed to stand for 20 min. in order to complete the reaction. Consequently the absorbance was read at 500 nm using UV-Visible spectrophotometer. Finally the concentration of tannin was calculated using D-Catechin standard curve (0, 0.2, 0.4, 0.6, 0.8 and 1 ml) or, (0, 12, 24, 36, 48, and 60 mg/100 g). The results were expressed as catechin equivalent of tannins in milligram per 100 g dry weight. The following formula was used to determine the tannin content of the samples.
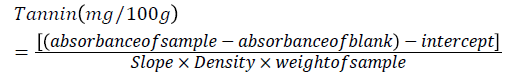
Statistical data analysis
The statistical data analysis method applied for this study was t-test of one population because of the study compared and analyzed the same samples of different zones to check whether or not the means were significantly different.
Antinutrient values of raw taro and yam
Food crops regularly eaten have many beneficial nutrients but there are traces of antinutritional components such as cyanoglucosides, oxalates, phytic acid, phenolics, protease inhibitors, heavy metal etc. These antinutritional factors when consumed in foods may have adverse effects on health through inhibition of protein digestion, growth, and Fe and Zn absorption [11]. The toxin however, is destroyed by processing techniques such as cooking, soaking and drying [14].
The Antinutrient contents of raw taro and yam samples from Benchmaji, Sheka and Keffa sites were presented in Tables 1 and 2 as follows.
| Proximate composition | Benchmaji raw taro sample (mg/100 g) Mean ± SD %RSD |
Keffa raw taro sample (mg/100 g) mean ± SD %RSD |
Sheka raw taro sample (mg/100 g) mean ± SD %RSD |
|||
|---|---|---|---|---|---|---|
| a,bOxalate | 0.085 ± 0.004 | 4.71 | 0.062 ± 0.0024 | 3.87 | 0.063 ± 0.005 | 7.93 |
| a,bPhytate | 37.62 ± 0.13 | 0.79 | 31.17 ± 0.12 | 0.38 | 161.13 ± 0.89 | 0.55 |
| a,bTannin | 6.72 ± 0.16 | 2.38 | 6.33 ± 0.23 | 3.63 | 4.18 ± 0.08 | 1.91 |
| aData were reported in dry basis, bMean value ± standard deviation, n=3 | ||||||
Table 1: Antinutrient values of raw taro samples from Benchmaji, Sheka and Keffa sites.
| Proximate composition | Benchmaji raw yam sample (mg/100 g) Mean ± SD % RSD |
Keffa raw yam sample (mg/100 g) Mean ± SD % RSD |
Sheka raw yam sample (mg/100 g) Mean ± SD % RSD |
|||
|---|---|---|---|---|---|---|
| a,bOxalate | 0.060 ± 0.004 | 6.67 | 0.0625 ± 0.003 | 4.80 | 0.054 ± 0.004 | 7.40 |
| a,bPhytate | 55.72 ± 0.63 | 1.13 | 65.40 ± 0.37 | 0.57 | 179.74 ± 1.34 | 0.75 |
| a,bTannin | 4.54 ± 0.11 | 2.42 | 3.06 ± 0.03 | 0.98 | 4.12 ± 0.06 | 1.46 |
| aData were reported in dry basis, bMean value ± standard deviation, n=3 | ||||||
Table 2: Antinutrient values of raw yam samples from Benchmaji, Sheka and Keffa sites.
As it has been depicted in Table 1, oxalate content of raw taro samples from all sampling sites were very small compared to the values of raw taro (156.33 mg/100 g) reported by Alcantara et al. [20] and the values of Anchote (6.56 mg/100 g) reported by Habtamu [21]. In the present study, the phytate contents of raw taro samples were higher than the values of raw Anchote (20.65 mg/100 g) reported by Habtamu [21] and lower than the values of taro (85.47 mg/100 g) reported by Alcantara et al. [20] with the exception of Sheka taro samples. The tannin contents of raw taro samples were lower than the values of raw taro (32.24 mg/100 g) and Anchote (50.19 mg/100 g) reported by Alcantara et al.; Habtamu [20,21]. Even though the raw samples had low antinutritional contents, it should be also minimized by cooking and boiling with suitable temperature before taking in to mouth [14].
As it has been indicated in Table 2, the oxalate content yam samples were very comparable with taro samples in all sampling sites and very small compared with the values of raw taro reported by Alcantara et al. [20] and the values of Anchote reported by Habtamu [21]. The phytate levels of yam samples on the other hand were lower than the values of taro (85.47 mg/100 g) reported by Alcantara et al. [20] with the exception of Sheka yam samples. The tannin contents of raw yam samples were lower than the values of raw taro (32.24 mg/100 g) and Anchote (50.19 mg/100 g) reported by Alcantara et al.; Habtamu [20,21].
As it has been depicted in Table 1 and 2; Figure 1, Sheka yam and Keffa taro were the least in oxalate and Phytate levels respectively whereas Sheka yam was the highest in Phytate contents. On the other hand, among all analyzed samples, Benchmaji raw taro samples were the highest in oxalate and tannin levels where as Keffa raw taro and yam samples were the least in Phytate and tannin levels respectively.
The percentage patterns of the six samples could be shown in decreasing order as follows: Oxalate: Benchmaji taro?Sheka taro?Keffa yam?Keffa taro?Benchmaji yam?Sheka yam; Phytate: Sheka yam?Sheka taro?Keffa yam?Benchmaji yam?Benchmaji taro?Keffa taro; Tannin: Benchmaji taro?Keffa taro?Benchmaji yam?Sheka taro?Sheka yam?Keffa yam.
Oxalate content
As it has been depicted in Table 1 and 2; Figure 1, the oxalate contents of raw taro and yam samples were lower than the values of raw taro (156.33 mg/100 g) reported by Alcantara et al. [20] and the values of Anclote (6.56 mg/100 g) reported by Habtamu [21]. Almost all sampling sites had lowest oxalate levels in comparison to the reported data in raw taro and Anclote samples. Even though the raw samples had low oxalate contents, it should be also minimized by cooking and boiling with suitable temperature before taking in to mouth [14].
Currently, patients are advised to limit their intake of foods with a total intake of oxalate not exceeding 50–60 mg per day [22]. The raw taro and yam tubers analyzed in this study were low compared to the recommendations for patients with calcium oxalate kidney stones. Traditional processing method like soaking and boiling before eating the tubers of both taro and yam tubers could have a positive impact on the health of consumers to enhance the bioavailability of essential dietary minerals of the tubers, as well as reduce the risk of kidney stones occurring among consumers.
Phytate content
Phytate is a salt form of phytic acid. Phytic acid acts as a strong chelator, forming protein and mineral-phytic acid complexes; the net result being reduced protein and mineral bioavailability [23].
The reduction of Phytate during processing through soaking and boiling of raw taro and yam tuber is expected to enhance the bioavailability of proteins and dietary minerals of the tubers and at the same time the lower level of Phytate may have some health promotional activities. Currently there is evidence that dietary Phytate at low level may have beneficial role as an antioxidant, anticarcinogens and likely play an important role in controlling hypercholesterolemia and atherosclerosis [24].
In the present study, the phytate contents of raw taro and yam samples were higher than the values of raw Anchote (20.65 mg/100 g) reported by Habtamu [21] and lower than the values of taro (85.47 mg/100 g) reported by Alcantara et al. [20] with the exception of Sheka taro and yam samples. In average, the daily intake of Phytate was estimated to be 2000–2600 mg for vegetarian diets as well as diets of inhabitants of rural areas of developing countries and 150–1400 mg for mixed diets [25]. Thus, in accordance with the daily intake of Phytate, all sampling sites had valuable Phytate amount, which had no health risk even at raw level. However, the Phytate contents can be reduced as a result of cooking and boiling with suitable temperature.
Tannin content
The tannin contents of raw taro and yam samples were lower than the values of raw taro (32.24 mg/100 g) and Anchote (50.19 mg/100 g) reported by Alcantara et al.; Habtamu [20,21].
The toxicity effects of the tannin might not be significant since the total acceptable tannic acid daily intake for a man is 560 mg per day [26]. Since the tannin content of raw taro and yam tuber was very low compared to its critical toxicity effect. Thus, tannin contents of the current study had no significant health hazard even at raw level. However, antinutrient in general, could be minimized as a result of cooking, soaking and drying [14].
Statistical data analysis
At the 0.05 level, the means of phytate were not significantly different in taro samples while the means of phytate were not significantly different in yam samples. On the other hand, the rest parameters’ means were significantly different in both samples. The influence of species, concentration of minerals in the soil and age of the plants may be the cause for the values obtained for similarity and difference in the samples under investigation (Table 3) [27].
| Parameters | tcal | |
|---|---|---|
| Taro | Yam | |
| Oxalate | 9.33 | 23.33 |
| Phytate | 1.81 | 2.52 |
| Tannin | 7.27 | 8.87 |
Table 3: T-calculated result of all parameters.
In this study, antinutrient of both taro and yam samples were compared and analyzed accordingly. Almost all sampling sites had lowest oxalate levels in comparison to the reported data in raw taro and Anchote samples. In accordance with the daily intake of Phytate, all sampling sites had valuable Phytate amount, which had no health risk even at raw level. However, the Phytate contents can be reduced as a result of cooking and boiling with suitable temperature. Since the tannin content of raw taro and yam tuber was very low compared to its critical toxicity effect. Thus, tannin contents of the current study had no significant health hazard even at raw level. However, antinutrient in general, could be minimized as a result of cooking, soaking and drying.
The authors express their sincere gratitude to the Mizan-Tepi University, Ethiopia for financing this research.