Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2018) Volume 8, Issue 4
Keywords: Intermittent fasting diet; Hypothalamus; Stereology; Mice
The high prevalence of obesity and overweight is a major public health problem affecting multiple chronic conditions in the world [1]. Obesity is the result of an imbalance between caloric intake and energy consumption [2]. Overweight and obesity may lead to increased risk of a number of chronic health conditions such as hypertension, diabetes mellitus, hyperlipidemia, gallbladder disease, cancer and osteoarthritis [1,3]. These issues have urged scientists to attempt to find diets that are suited to modern lifestyles and may also hinder the growing trend of such diseases [4,5]. Intermittent fasting diet (IFD), also referred to as every other day feeding, is an intermittent feeding or dietary intervention, which involves feeding cycles and fasting with reduced to 0% caloric intake for 24 hours [6,7]. As the daily energy intake in the IFD is less than that in the normal diet [8], this issue causes a weak biological stress on the body, which, in turn, results in the activation of the immune response for protection against damaging factors. Studies showed several metabolic changes occur during this fasting period such as decreased glucose levels, decreased glycogen stores, fatty acid mobilization, and decreased leptin [4-11]. Additionally, this feeding protocol results in increased lifespan of animals and decreased obesity and metabolic disorders associated with age-related diseases in humans [7,10,11]. Neuroprotective effects of IFD were also observed in several experimental studies. Previous studies have shown that neurons in rats or mice’ brains receiving an IFD are at a lower risk for death by oxidative, metabolic and excitotoxic insults [5,7,9,11-15]. Also studies in mammals showed the IF is an effective diet in protecting the hippocampal neurons against excitotoxic injury. Reduction in oxidative stress and increased hippocampal neurogenesis consider as neuroprotection mechanisms of IFD in the mice hippocampus [5-9,11,13]. Additionally, several studies have investigated the effects of this diet on functional changes and neurodegenerative problems such as dementia associated with aging, Alzheimer’s, Huntington’s and Parkinson’s disease as well as epilepsy and mood disorders [4,6,11,16].
As energy balance, feeding behavior and body mass are primarily controlled by distinct nuclei within the hypothalamus such as the arcuate, ventromedial, dorsomedial and paraventricular [17,18] and because destructing hypothalamic regions can cause several eating disorders such as hyperphagia or reduced food intake [18,19]; therefore evaluation of the efficiency and potential effects of the IFD on the hypothalamus as the main appetite and energy balance controller in the body is valuable and necessary. Therefore, owing to the fact that little is contributed to the literature concerning the potential effects of IFD on the hypothalamus and with regard to the paucity of research on the hypothalamic histomorphometric parameters in mammals under the IFD, we employed a stereological method, as an efficient and unbiased technique to quantitate structures, to determine the potential effects of short- and long-term reductions in food intake using IFD on the histomorphometric parameters such as volume, number and density of neurons in adult male mice hypothalamus. In addition, measurements of body weight and biochemical parameters were done to confirm the effectiveness of IFD on the mice.
Sampling
Forty male Balb-c mice, approximately eight weeks of age and average weight of 25 ± 2 g were housed at 22 ± 1°C under a 12/12 h light/dark cycle. For the experiments mice were taken randomly from cages. All the procedures were approved by the Ethics Committee of Kerman University of Medical Sciences, Kerman, Iran.
Study groups
The animals were randomly divided into four different groups (n=10 per group) after acclimatization in order to short- and long-term normal diet (ND) groups that received the normal diet (ad libitum) for four and eight weeks respectively, and Short- and long-term IFD groups that had ad libitum access to food on alternating days (i.e., every other day fasting) for four and eight weeks respectively. Other conditions of the animals remained unchanged and according to the standard. Body weight was measured at the end of the experiment, while food intake was measured daily. Finally, the mice were deeply anesthetized with chloroform and sacrificed after collecting their blood sample from the heart. The blood plasma was used to estimate the blood sugar (BS), low density lipoprotein (LDL), cholesterol (CL) and triglyceride (TG). To remove the brains, mice were cardiacally perfused with 0.09% saline and 4% buffered formalin.
Tissue preparation
All brain samples were immersed in 4% buffered formalin solution immediately and remained there for 24 hours. Thereafter, the samples were processed for paraffin embedding and blocked. The samples were sectioned coronally with the determined thickness of 30 μm and constant distance of 180 μm and stained by Kluver-Barrera (combination of Luxol fast blue and cresyl violet).
The Sections and microscopic fields were sampled using the systematic uniform random sampling (8-10 sections per hypothalamus were obtained serially).
The anatomical classification of the hypothalamus
The hypothalamus in the prepared and stained slides was outlined according to Paxinos and Franklin’s mouse brain in stereotaxic coordinates. The reviews performed found to range from Bregma -0.34 mm to Bregma -2.70 mm in images from Paxinos’s atlas, pages 30 to 78, belonging to the hypothalamus [20].
Stereological volume estimation
For determining the total volume of the hypothalamus, the Cavalieri technique and point counting grid were used. The slides were studied with E200Nikon Eclipse microscope and the object lens × 1. The images taken by the camera (Sony Color Video Camera) were transferred to the computer and studied with StereoLite software (Shiraz University of Medical Sciences, Iran). The calibrated graticule was used for determining the magnification scale of the images. The points falling on the image of each section were counted at the final magnification of 45x (Figure 1). Total volume obtained from the equation 1:
VΣ =p. a (p). D
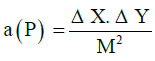
Σ p = total points
a (p) = range of points
M = magnification
ΔY= length of two points
ΔX = width of two points
D = distance between sections
The CE for the Cavalieri’s estimator of volume was 5.7% when the cross-sectional areas “ΣA” were estimated by the software.
Estimating the number of cells (the Optical Disector technique)
The counting tool in the Optical Disector is a frame, which, in its simplest form, is composed of two sides as the authorized level (top and right sides) and two sides as the level of illegal (left and bottom sides) (Figure 2). To estimate the mean numerical density of neurons in the hypothalamus, the equation 2 was used:
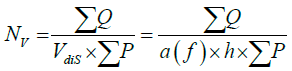
NV = number of density per unit volume (mm3)
a (f) = frame area (mm2), (0.037 × 0.037)
h = height of disector (mm), (0.015)
ΣQ = set of neurons counted
ΣP = total points with frame which is faced with reference area
To estimate the total number (N) of neurons in the mice hypothalamus, the numerical density (NV) was multiplied by the volume of the hypothalamus or the reference volume (Vref). The equation 3:
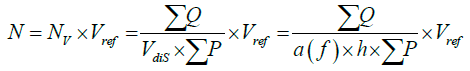
The neuronal nuclei were counted in 8-10 sections, 70-75 optical disectors, and 495-515 neurons per hypothalamus in each mouse, providing a mean CE of 7% on the estimates of the total number.
Statistical Analysis
The data were evaluated using SPSS version 19.0 for Windows. The Kolmogorov-Smirnov test was used to evaluate the normality of the data, and one-way analysis of variance (ANOVA) followed by LSD Post-hoc test was performed for statistical analysis. P values <0.05 were considered as significant. The experimental values were expressed as mean ± standard error of mean (SEM).
Body weight and biochemical parameters
In this study, the effects of short-term (4 weeks) and long-term (8 weeks) exposure of adult male mice to the IFD compared to the ND were evaluated.
The results showed that the average body weight of the short-term IFD group was significantly reduced to 10% of the body weight of the short-term ND group (P<0.01). Furthermore, there was a significant decrease by approximately 12% in the body weight of the mice receiving long-term IFD compared to those receiving long-term ND (p<0.01) (Figure 3).
Statistical analysis showed a significant difference in blood factors (BS, CL, TG, LDL) in both short- and long-term treatment with IFD in comparison with control groups (p<0.05); (Table 1).
| Groups | Blood Sugar | Triglyceride | Cholesterol | LDL |
|---|---|---|---|---|
| Short Term ND | 130.7 ± 0.74 | 211.4 ± 4.21 | 172.4 ± 3.35 | 66.4 ± 2.3 |
| Short Term IFD | 121.4 ± 1.11* | 184.6 ± 3.9* | 158.6 ± 2.3* | 57.4 ± 1.71* |
| Long Term ND | 130.4 ± 0.8 | 209.1 ± 4.2 | 176.7 ± 2.6 | 70.4 ± 1.39 |
| Long Term IFD | 120.4 ± 1.15** | 187.6 ± 3.9** | 158.7 ± 2.8** | 59.2 ± 1.25** |
Abbreviations: ND: Normal Diet; IFD: Intermittent Fasting Diet; LDL: Low Density
Lipoprotein
Data are shown as Mean ± SEM (standard error of mean); n=10 in each group
*Significant difference with short term ND group (p<0.05)
**Significant difference with long term ND group (p<0.05)
Table 1: Blood factors (mg/dl) in the mice were fed with ND and IFD for short and long term periods.
Stereological analysis
The total volume of the hypothalamus was assessed. Furthermore, the numerical density and total number of neurons in the mice hypothalamus were determined. Although the total number of neurons in the mice hypothalamus appeared to increase in the short and long-term treatment in the IFD groups, no statistical significant difference was observed as compared to the ND groups. The CE for the number estimation was 0.06-0.08. Moreover, no significant changes were identified in the total volume of the hypothalamus in the short-term IFD group compared with the short-term ND group; however, significant increase in the mice hypothalamus total volume in the long-term IFD group compared with the long-term ND group (p<0.03) was shown. The CE for the volume estimation was 0.05-0.09.
In regards with the numerical neuronal density of the hypothalamus, statistical analysis showed no significant difference in the short-term IFD group compared with the short-term ND group and in the long-term IFD group compared with the long-term ND group. Although the numerical density appeared to increase in the short-term IFD group and decrease in the long-term IFD group (Table 2).
| Groups | Neuron number | Volume (mm3) | Numerical density (neuron/mm3) |
|---|---|---|---|
| Short Term ND | 663904.4 ± 21452.2 | 2.25 ± 0.06 | 293272.4 ± 8222.42 |
| Short Term IFD | 684710.5 ± 19482.95 | 2.3 ± 0.09 | 303398.7 ± 13955.92 |
| Long Term ND | 661306.3 ± 17337.8 | 2.27 ± 0.07 | 294095.4 ± 13071.1 |
| Long Term IFD | 709544 ± 6649.04 | 2.53 ± 0.1* | 284342.9 ± 11315.7 |
Abbreviations: ND: Normal Diet; IFD: Intermittent Fasting Diet
Data are shown as Mean ± SEM (standard error of mean); n=10 in each group
*Significant difference with long term ND group (p<0.03)
Table 2: Total number of neurons and volume and numerical density in the mice hypothalamus, which were fed with ND and IFD for short and long term periods.
According to the World Health Organization (WHO), approximately 39% of the world population aged between 18 and over are estimated to be overweight and 13% were obese [21]. These outcomes leave a huge burden on society and economy driving extensive research being done on developing dietary treatments that decrease overweight and obesity rates [8].
In the present study, biochemical and body weight analyses confirmed the efficiency of IFD and results showed that short- and long-term reductions in food intake can improve histomorphometric changes in the hypothalamus.
Previous experiments showed that Sprague–Dawley rats with the every-other-day fasting diet, consume approximately 30% less food over time and have body weight of 10-15% below than that of rats fed ad libitum [22]. In this study, we observed approximately 10% reduction after 4 weeks and 12% reduction after 8 weeks in the body weight of the IFD mice compared to the ND control mice. Increase of the interval between meals results in reduced circulating leptin levels and weight loss and thus, is often adopted as an energy balance between calories consumed and calories expended [17]. In previous studies the IFD decreased insulin levels, FBS and TG by nearly 10%, as compared to the normal diet, and increased insulin sensitivity and High density lipoprotein (HDL) levels in blood [4,6,9,10,23]. Similar to these studies, our findings showed that the IFD caused a reduction in blood factors in mice. This reduction was observed in the fourth week of the short-term intervention and continued until the eighth week of the long-term regimen, indicating the immediate effect of the IFD on blood parameters.
Some studies used stereological techniques to estimate the volume and number of neurons in brain regions such as the hippocampus, spiral ganglion, cingulate cortex and neocortex [24-26]. The present study used the same technique as applied in the mentioned studies. Results showed that the total number of neurons and total hypothalamus volume increased in the short- and long-term IFD groups, as compared to the short- and long-term ND groups.
Studies of mammalian brain have shown that postnatal neurogenesis is conserved in the adult hypothalamus. Intraventricular injection of 5-Bromo-2΄-deoxyuridine (BrdU) has shown that in this region a significant number of new cells are continuously generated [18,19]. Brain plasticity enhancement by IFD has been suggested by numerous studies. Manzanero et al. revealed following energetic challenges such as exercise and IFD, adult neurogenesis rates increase in the intact brain [16]. Additionally, Tajes et al. showed that some death signals implicated in neuronal death and aging processes are reduced under the influence of this dietary regimen [13]. Also, several experiments on the mice hippocampus showed the IFD leads to enhanced neurogenesis and gliogenesis, as well as decreased cell death rate and increased newly born cells survival in the dentate gyrus (DG) [5,11,14,15,27]. Moreover, hippocampal neurons resistance to chemically induced degeneration and experimental models of stroke in rats increases when receiving an IFD [6,11,14,16].
Similarly, Djordjevic et al. reported that synaptophysin expression in DG and CA3 was enhanced by long-term dietary restriction [28]. In a similar vein, caloric restriction (CR) leads to age-related declines in synaptophysin levels [13], indicating that synaptic functionality preserved by IFD and CR regimens is associated with reduced hippocampus stress level, enhanced synaptic plasticity, and increased neurogenesis [27-30]. Studies on the IFD neuroprotective mechanism suggest that a mild adaptive cellular stress response is induced, which causes neurons to upregulate the expression of neurotrophic factors and protein chaperones such as heat-shock protein 70 [11,13-15]; therefore some of the beneficial effects of IFD on the brain are mediated by brain-derived neurotrophic factor (BDNF) and synaptophysin signaling; these factors are associated with neurogenesis and brain plasticity in various brain regions such as the lateral ventricles and hippocampus [6,9,15,31].
The numerical density was also shown to increase in the short-term IFD and decrease in the long-term IFD group. Since numerical density is a relative estimate of number and volume, like all ratios, it is affected by changes in both parameters [32]. Therefore, a slight increase or decrease in each of the parameters (number and volume) causes a variation in the numerical density.
In the short-term IFD, the numerical density increased due to an increase in the number of neurons. While in the long-term IFD, the volume increase was more significant and the numerical density decreased in response.
The findings in our study showed an increase in total hypothalamus volume with no changes in total number or density of neurons after long-term IFD, which leads to speculations that the observed hypothalamic hypertrophy could be the results of increases in synaptic number, myelination or other morphometric changes in the cellular or intracellular spaces; although, further studies are needed to examine the mechanisms (e.g., increased neurogenesis and neuronal synaptic plasticity) related to these alterations. In addition, the positive impacts of short- and long-term IFD on body weight and blood factors were observed which all could reflect a healthier condition.
This article was a part of the thesis written by Azam Hassanpour, a PhD candidate in department of Anatomical Sciences and was supported by Kerman University of Medical Sciences, (grant numbers 45-94, 2015). The authors would like to acknowledge the Histomorphometry and Stereology Research Center at Shiraz University of Medical Sciences, Shiraz, Iran.