Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Environmental pollution caused by pesticides and ultra-trace amounts of inorganic ions in the different environmental samples has become an issue of serious concern in the last years. Thus, the development of novel, simple, fast, sensitive, and cheap methods for the detection of pesticide residues and ultra-trace inorganic ions in the environmental sample is very significant task now days. The SWAdSV can be conjuncted with different modified electrode such as SWAdSV with modified Hanging mercury drop electrode, SWAdSV with multi-walled carbon nanotube paste electrode, SWAdSV with boron-doped diamond electrode, SWAdSV with flow-injection analysis detection and etc. SWAdSV can be used to perform an experiment much faster than normal and differential pulse techniques, which typically run at scan rates of 1 to 10 mV/sec. Square wave voltammetry employs scan rates up to 1 V/sec or faster, allowing much faster determinations. A typical experiment requiring three minutes by normal or differential pulse techniques can be performed in a matter of seconds by square wave voltammetry. The aim of the review was the use of square wave adsorptive stripping voltammetry in conjunction with different modified electrode for the determination of pesticide and ultra-trace inorganic ions in environmental samples.
SWAdSV, HMDE, pesticide, ultra-trace inorganic ions
In the agricultural activity, different pesticides and other classes of chemical products is used to achieve greatest yield. This may cause various serious impacts on the natural environment, causing an increased level of pollutant residues in water, soil, river sediments, and foodstuffs (Skrzypczy´nska et al., 2016). When pesticides used properly, it can save up to 40% of crop losses. There are more than 1,000 pesticides used globally on soil and crops. However, when pesticides are misused, the consequences of environmental and public health can be very serious. The increased risk of pesticide residues and ultra-trace inorganic ions accumulation can cause serious health problems and also exposure human through their remnants present in the food. In addition to this, different pesticides, ultra-trace inorganic ions and other classes of chemical products include active ingredients and inert ingredients that may be carcinogens or toxic substances (Figueiredo-Filho et al., 2015). Prevention of the negative effects of these pesticide, ultra-trace inorganic ions and chemical products requires a systematic control of content of their remains in agricultural products, food, soil, and water. For instance, urea herbicides are among the most widely used in the culture of trees, and their action is very similar to the triazine pesticides. The toxicity effects of pesticides and herbicides on fish and invertebrates have been studied. It was observed that concentrations in the order of parts per million affected the embryonic development (Dariusz et al., 2016).
The elaboration of modern, easy to operate, rapid, sensitive, and cheap methods for the detection of hazard residues in the environment is a main task in electro-analytical chemistry. It has been widely used for the determination of electro-active compounds due to their simplicity, Sensitivity, stability, and low cost effects (Svorc, 2013). The electro-analytical techniques have long history in applications towards analysis of biocides, pesticides and ultra-trace inorganic ions in different environmental matrices. Many published articles report the pollutants determination in natural samples, mainly using separation and spectrometric methods (Masi´a et al., 2014). Among the pesticides used, nitrothal isopropyl (NT) is a selective pesticide applied towards control of the development of many side effects of diseases caused by microorganisms in plant breeding of agricultural crops has been studied. NT is classified as a third-class toxicity pesticide for mammals. It is also applied as a seed treatment, in order to combat diseases of vegetable and ornamental plants (Dariusz et al., 2016).
The most common methods of pesticides determination employ gas, liquid or thin-layer chromatography and selective detector (Deutsche et al., 2006), (Zhong et al, 2009). Voltammetry techniques are described with several advantages like wide linear concentration range, good sensitivity, low apparatus cost, capability of miniaturization, capability for on-site detection, relatively short time of analysis, and insensitivity to matrix (Smarzewska, 2016). Therefore, reliable analytical procedures needed for their determination. As a result, the development of analytical methods to monitor different types of pesticides and ultra-trace inorganic ions in biological or environmental samples is fundamentally important. There are a lot of papers reporting the determination of pesticides in environmental and agricultural samples, mainly using spectrometric and separation methods (Lezi & Economou, 2015). Nevertheless, many of them have some drawbacks, i.e. long-time of analysis, tedious preparation of samples, time-absorbing and complicated pretreatment steps, as well as the necessity of the use of considerable amounts of expensive and toxic solvents. The alternative to aforementioned analytical methods is voltammetry, which is especially adequate for large-scale monitoring of electrochemically active environmental pollutants (Wang, 2000).
On the other hand, the determination of trace concentration levels of ultra-trace inorganic ions is of interest in different fields such as environmental surveillance, food control and occupational hazards. SWAdSV has received increasing attention as a technique for ultra-trace quantitative analysis because it can be used to perform an electro-analytical measurement faster than conventional differential pulse voltammetry and with higher sensitivity (Ali et al., 2012). The applicability of the SWAdSV method to the analysis of foodstuff was assessed by the determination of ultra-trace inorganic ions content in environmental samples. SWAdSV procedure is well suited for the determination of ultra-trace amounts of inorganic ions through the adsorbed complexes. In adsorptive stripping voltammetry approach, the metal ion of interest is absorbed on the working electrode by means of a non-electrolytic process prior to the voltammetric scan. By this electroanalytical technique, the analytes are determined as complexes with different organic ligands adsorbed on the electrode surface (Alghamdi, 2010).
Square wave stripping voltammetry
Square wave stripping voltammetry is first pre-concentrate the analyte on the surface of the electrode and then strip the analyte and measure.
Square wave stripping voltammetry is a potential alternative for ultra-trace analyses and pesticides due to numerous advantages such as faster analysis, higher selectivity and sensitivity, low cost, easy operation, and possibility to perform the analysis (Nguyen et al., 2019).
There are different square wave stripping voltammetry such as anodic stripping, adsorptive stripping, cathodic stripping (Guziejewski et al., 2011) and abrasive stripping voltammetry. In adsorptive stripping voltammetry method, accumulation potential (Ead), accumulation time (tad), and pulse amplitude affect the sensitivity and selectivity of the method.
The Ead is applied to the working electrode and makes the electrode surface negatively charged or positively charged (Thuong et al., 2019).
Many organic species such as nitrothal-isopropyl, heme, chlorpromazine, codeine, and cocaine and ultra-trace inorganic ions and etc. have been determined at micromolar and nanomolar concentration levels using AdSV. The adsorbed species is quantified by using a voltammetric technique such as DPV or SWV in either the negative or positive direction to give a peak-shaped voltammetric response with amplitude proportional to concentration.
Investigation of nitro-group containing compounds using voltammetric methods has been utilized since the very beginning of polarography and voltammetry. The optimal square wave technique parameters are pulse frequency, amplitude, and step potential. Accumulation time and accumulation potential are studied to select optimal conditions in adsorptive stripping voltammetry (Dariusz et al., 2016).
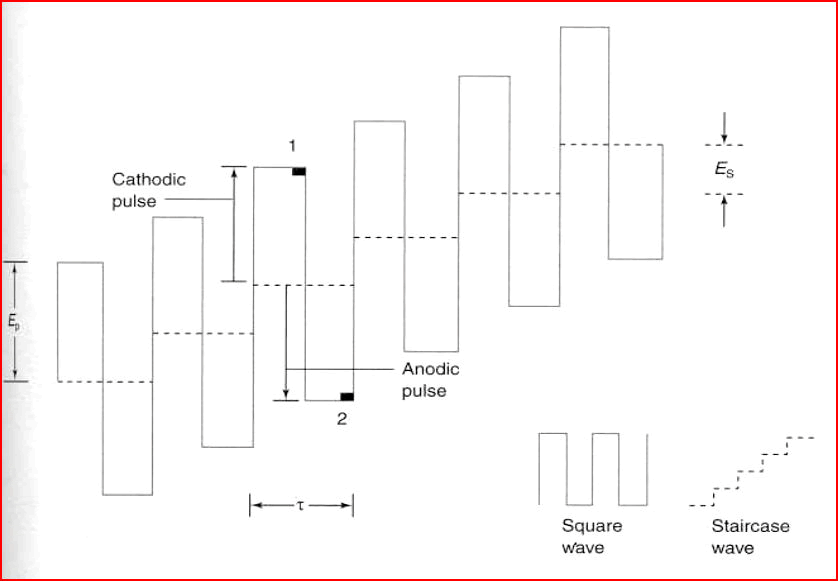
Figure 1: The optimal SW technique parameters.
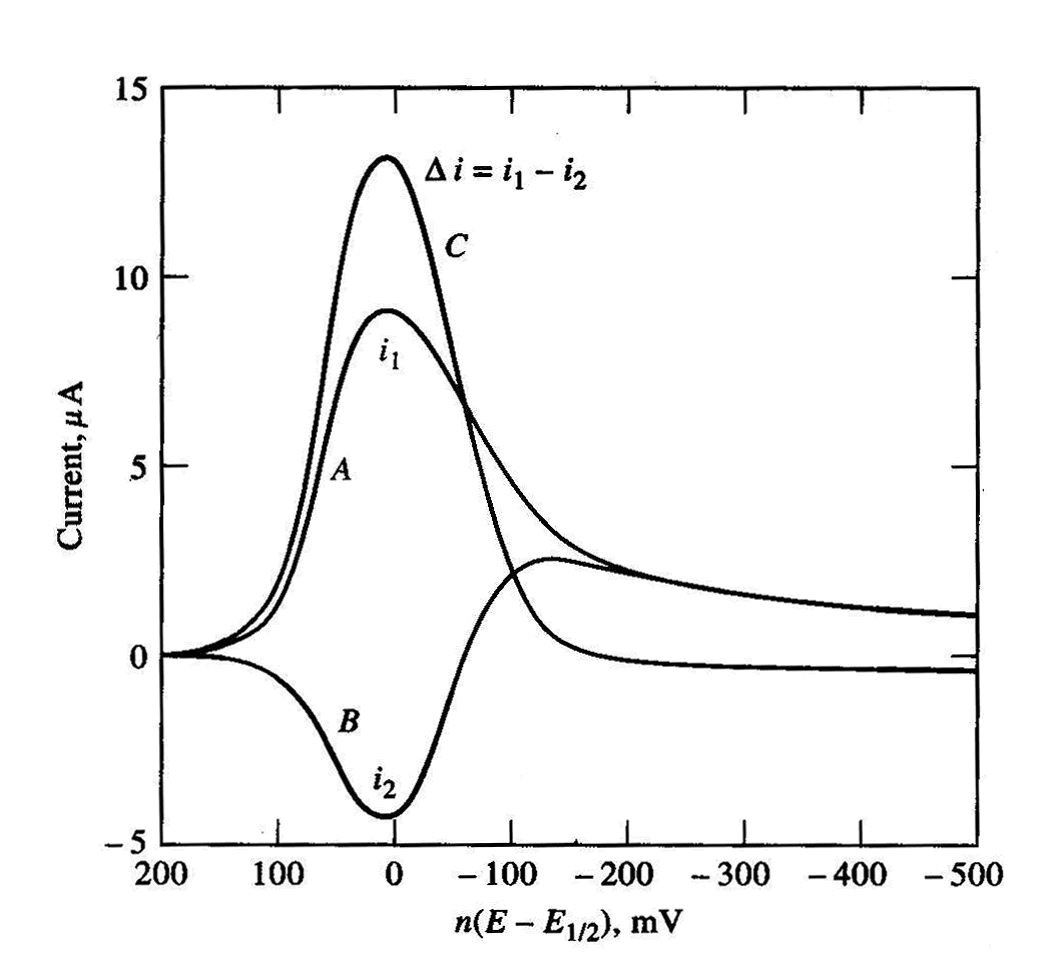
Figure 2: SWAdS Voltammograms.
SWAdSV with modified hanging mercury drop electrode
Hanging mercury drop electrode commonly used for characterization of electrode processes that can be characterized with wide range of potential in a negative region, easily renewable and smooth surface, capability for preconcentration of analytes, and so forth . Based on the adsorption character of investigated compounds, onto the surface of the HMDE, validated, simple, fast and sensitive SWCASV procedure for quantification of 3-Hydroxyflavone, Morin and Hesperidin compound under investigation in bulk form and in biological fluids was studied. Result from this study reveals that the application of SWCASV mode for trace analysis of three flavonoids in presence of Cu(II) is more sensitive than that in the absence of Cu(II) (Yassien et al., 2011). Until very recently, mercury electrodes were typically used in a stripping voltammetry as the working electrodes to determine the trace level of metal ions and electrochemically reducible organic compounds. Nevertheless, due to toxicity of this element, there is a tendency to replacement of mercury with other less or nontoxic materials. However, the high toxicity of mercury in combination with the inconvenience of its handling is becoming the main drawback of mercury-based SWAdSV. For this reason, various types of working electrodes such as platinum, silver (Piech et al., 2016) & (Peckov´a et al., 2009), or iridium have been proposed as an alternative to mercury.
The pesticide diazinon was determined in its insecticidal formulations by SWAdSV. For mercury electrodes, there is a point on the surface of the electrode that is not charged depending on the solution, which is called a “capillary electrical zero point”. Before that point is a positive charge and after that point is a negative charge, and the adsorption ability is affected by the applied electrode potential value (Dariusz et al., 2012). In addition, the adsorption process is influenced by other specific factors that have a chemical specific affinity for mercury such as sulfur and nitrogen compounds. Due to high hydrogen over potential mercury-based working electrodes were found suitable for voltammetric determination of nitro-aromatic molecules.
Despite the increased fears of liquid mercury toxicity in the past years, a stable attention is given to the development of electroanalytical methods that utilize hanging mercury drop or amalgam electrodes . One of the electroanalytical method, square-wave voltammetry (SWV), is considered to be a very fast and highly sensitive, and moreover, the most frequently used technique for analytical purposes (Mariola et al., 2016).
Figure 3: The Hanging Mercury Drop Electrode (HMDE).
SWAdSV with multi-walled carbon nanotube paste electrode
The morphological, structural and electrochemical characteristics of the nanostructured material were evaluated using spectrophotometry, X-ray diffraction, transmission electronic microscopy and voltammetry (Anderson et al., 2019). The amazing and unique properties of carbon nanotubes such as high electrical conductivity, high surface area, good mechanical strength and excellent thermal and chemical stability have received intense attention in the field of new electrochemical sensors and biosensors . The sufficiently good recoveries, HPLC comparison results, and low relative standard deviations reflect the high accuracy and precision of the proposed voltammetric method. A novel electro-analytical method involving SWSV at MWCNTPE was proposed to determine cyromazine content in agrochemical formulation and natural water. The SWS voltammetric method presented for the quantitative determination of cyromazine allowed the accurate determination and was found to be rapid, simple, and highly sensitive . The main advantage of such a procedure is the possibility to determine the concentration of the active component directly from the fungicide formulation and natural samples without any previous treatment, such as extraction, clean-up, derivatization, or pre-concentration, which are tedious, time consuming, and also polluting. Nevertheless, the major problem for developing carbon nanotube based sensors is their low solubility in solvents such as ethanol, methanol, isopropanol and water (Jorge et al., 2014).
SWAdSV conjunction with boron-doped diamond electrode
The boron-doped diamond is a relatively new material used for preparation of solid electrodes. Characteristics of boron-doped diamond electrodes are the widest usable electrochemical potential window among all metal and carbon electrode materials (about 3.5 and 5 – 7.5 V in aqueous and non-aqueous organic solutions, respectively), low and stable background current, high resistance to deactivation by surface fouling, corrosion stability in highly aggressive media, low adsorption of contaminants, relative insensitivity to dissolved oxygen, good mechanical robustness. Due to that, this electrode plays an important role in electroanalytical chemistry and is applied in different field nowadays. However, it is important to underline that, for many analytes, these properties of the boron-doped diamond electrode are extremely dependent on its surface termination which can be modified by appropriate electrochemical pre-treatment (anodic or cathodic) or mechanical treatment (Aydın et al., 2020).
SWAdSV with Flow-Injection Analysis Detection
SWAdSV and flow injection analysis-SWAdSV analytical methods have been developed for the determination of analyte in samples. An alternative method for fluoxetine quantification in pharmaceutical products has also been developed by including a SWAdSV detection system in flow injection analysis system. This method enables analysis of up to 120 samples per hour at reduced costs. The flow injection analysis with SWAdSV method has the advantage of high sample throughput, significantly reducing the analysis time and costs (Henri et al., 2007).
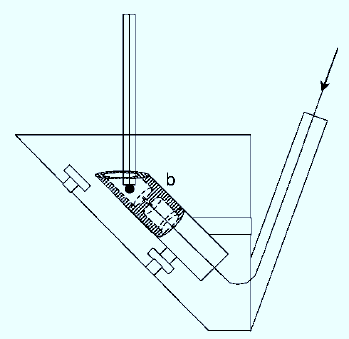
Figure 4: SWAdSV detection system in an FIA system.
Voltammetric procedure
The general procedure used to obtain square wave adsorptive stripping voltammograms was as follows: the supporting electrolytes have to be prepared (buffer mixed with water) and placed in the voltammetric cell. Then the solution is purged with argon or with nitrogen for few minutes with stirring. Prior to the recording the voltammogram, oxygen is removed by a pure nitrogen stream (Thuong et al., 2019). Next, the accumulation step at a constant potential is applied. At the end of the accumulation period, the stirring is stopped and the solution is equilibrated for few minutes. Following the equilibrium step, a negative on going potential scan is applied. If some reagents were subsequently added, the test solution is purged with argon for further 30s. The reported signal is measured after subtracting of the blank solution. All measurements are performed at room temperature. (Dariusz et al., 2012).
Validation of the method
The SWAdSV method is validated interms of stability, linearity, sensitivity, precision, accuracy, recovery, selectivity, robustness, and ruggedness (Incilay et al., 2009).
Stability
A standard stock solution of analyte under study is kept in the dark at low temperature. The stability of analyte standard stock solution is tested for one month and it is found to be stable during this period. Under the optimum conditions, the short time stability of analyte standard solution is evaluated by the proposed method. No changes observed in the peak potential and peak current over a period of 8 hour to predict its stability.
Linearity
The linearity is defined as the ability of the method to obtain test results that are directly proportional to the concentration of the analyte within a given range. The range is the interval between the high and low levels of analyte studied. Under the optimum conditions, SWAdSV voltammograms recorded with increasing amounts of analyte showed that the peak current increased linearly with increasing concentration.
The parameters of the concentration-peak current straight line were calculated by the least-squares method. The linearity is checked by preparing standard solutions for 10 different concentrations for the proposed SWAdSV method (Luisa et al., 2017).
Sensitivity
The sensitivity of the developed method is checked in terms of limits of detection (LOD) and quantitation (LOQ) values. The LOD is defined as the lowest concentration of an analyte in a sample which can be detected. The LOQ is defined as the lowest concentration of an analyte in a sample which can be determined quantitatively with an acceptable level of accuracy and precision under the optimum conditions of the method [14]. SD is the standard deviation of y-intercepts of regression lines and b is the mean slope of the calibration curves. These LOD and LOQ data indicate that the proposed method is sensitive for the analysis of analyte under study. In order to enhance sensitivity of the determination, various parameters such as pH, buffer concentration, frequency, amplitude, step potential, accumulation time, and potential must be investigated (Manuela et al., 2011).
Precision
Precision is defined as the closeness of agreement between independent test results obtained under optimum conditions and is normally expressed as the percentage relative standard deviation (RSD%). The precision of the proposed method was investigated with respect to repeatability and intermediate precision.
Accuracy and recovery
Accuracy is defined as the closeness of the test results obtained by the analytical method to the true value. It is determined by calculating the percentage relative error (bias %) between the measured mean concentrations and added concentrations. The accuracy of the proposed method was also tested by recovery experiments. For this purpose, the recovery test is performed by the standard addition method. For this purpose, appropriate volume of tablet solution was added to supporting electrolyte. After the voltammogram was recorded, three known concentration levels of analyte standard solutions were added and voltammograms were recorded after each addition (Tuba et al., 2014)
Selectivity
Selectivity is defined as the ability of the method to determine the analyte response accurately and specifically in the presence of other components of a sample matrix under the stated conditions of the analysis. Comparison of the recorded voltammograms obtained from analyte under study and standard solutions in the same concentration showed that the peak potential and peak current of analyte did not change.
Robustness
The robustness of the method shows the reliability of an analytical method with respect to small, but deliberate variations in method performance parameters. These parameters including pH of supporting electrolytes, Eacc and tacc were investigated for concentration of analyte. Consequently, this SWAdSV method was reliable for the analysis of analyte and the proposed method could be considered robust.
Ruggedness
The ruggedness of the proposed method was evaluated by applying the developed procedure to assay of concentration of analyte using the same instrument by two different analysts under the same optimized conditions at different days. The results were compared by means of the T and F-tests. Since there was no significant difference between the results obtained by the two analysts, the proposed method may be considered rugged.
SWAdSV method application
Square wave adsorptive stripping voltammetry method is applicable in pharmatecal industry, detection of ultra-trace metal ions, pesticides, herbicides, fungicides in the environmental samples (Dariusz et al., 2012). Electro-analytical techniques are also advantageous due to characterization possibilities in kinetic and equilibrium studies (Mirceski et al., 2016).
Moreover square wave voltammetry is recognized as most frequently used voltammetric technique for electroanalysis. Electrochemical techniques are characterized by several advantages, i.e. a wide linear concentration range, relatively high sensitivity, lower apparatus cost, the miniaturization capability, suitability for real-time detection, short analysis time, as well as less sensitivity to matrix effects in comparison with other methods. It can be concluded that this SWAdSV method can be successfully and reliably applied to the routine analysis in quality control laboratories for the analysis of different pharmatical tablets, pesticides and ultra-trace in organic ions. The method is simpler, faster (requiring <2 min to perform) and requires less expensive equipment than chromatographic methods (Bengi & Sibel, 2011).
A review on square wave adsorptive stripping voltammetry determination of pesticide and ultra-trace inorganic ions in the environmental samples has been reviewed. Square wave adsorptive stripping voltammetry is a potential alternative for ultra-trace analyses and pesticides due to faster analysis, higher selectivity and sensitivity, low cost, easy operation, and possibility to perform the analysis and therefore it can be used for determination of pesticides and ultra-trace metal ions in the environmental samples with no matrix effects on the measurable response. All the data received from many previous report using the optimized experimental conditions and voltammetric parameters acknowledged the practical application and viability of the proposed methodology, ensuring a new instrument for quantification of pesticide and ultra-trace inorganic ions in environmental samples. The use of SWAdSV is usually more efficient than other conventional techniques. The newly developed procedure allows accurate detection of pesticide and ultra-trace inorganic ions introduces a simple, fast, selective, and highly sensitive methodology. The capability to determine the pesticide and ultra-trace inorganic ions content directly from the matrix medium or natural samples without any laborious pretreatment which are usually time-consuming and environmentally unfriendly is one of the main advantages of the method.
Published: 06-Sep-2021 , DOI: 10.35248/2155-9600.21.s7.1000173
Copyright: This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.