Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Background: Refeeding syndrome (RS) is a serious clinical syndrome, its early identification is key to safe management of adolescent anorexia nervosa. The aim of this study was to evaluate existing practices in a highly specialist centre for eating disorders and compare refeeding management, nutritional, and clinical outcomes in cases admitted to a medical stabilisation unit with those managed in outpatient care. Methods: Retrospective analysis of electronic case records of 59 adolescent patients at high risk of developing refeeding syndrome and treated for anorexia nervosa by a specialist eating disorder centre in London over a 5-year period. Statistical analysis compared refeeding methods used in this population to establish if there were differences in refeeding methods used between the inpatient and outpatient groups. Results: The inpatient group (n=19) had significantly lower baseline energy intakes, prior to assessment (374 kcal/d ± 205 compared with 621 kcal/d ± 348, p=0.001) and higher rates of weight loss at leading up to assessment (0.86 kg/week ± 0.7 compared with 0.38 kg/week ± 0.7, p=0.003), than the outpatient group (n=40). Incidence of RS symptoms did not differ significantly between groups. Conclusions: These findings support recent evidence that advocates a less conservative refeeding approach for moderately malnourished adolescents with AN and a review of current national guidance.
Nutrition; Refeeding management; Adolescent anorexia nervosa; Eating disorders
(RS) Refeeding Syndrome; (AN) Anorexia Nervosa; (MARSIPAN) Management of Really Sick Patients with Anorexia Nervosa; (MCCAED) Maudsley Centre for Child and Adolescent Eating Disorders; (CAMHS) Child and Adolescent Mental Health Service; (BMI) Body Mass Index
Refeeding syndrome (RS) can cause ill health and even death. It can occur when nutrition is reintroduced to malnourished patients. This study aims to compare management of RS in two different treatment settings for adolescents with anorexia nervosa: hospital and outpatient care, in terms of outcomes measures such as blood results. This was achieved by looking back over 5 years of patient records and by using statistical methods to compare patients who were treated in hospital with those who were treated solely as outpatients. The study found that, for most adolescents with anorexia nervosa, less conservative refeeding practices are safe. Results indicate that a review of current national guidance could be helpful.
Refeeding syndrome (RS) is a serious and potentially fatal clinical condition that can occur when refeeding malnourished patients [1]. Cautious approaches to refeeding have historically been advocated in low weight adolescents with restrictive anorexia nervosa (AN) [2]. However, in more recent years, there has been a growing body of evidence supporting less conservative refeeding practices in this group [3-5]. Inconclusive evidence on how to safely refeed malnourished patients has resulted in widely variable and inconsistent refeeding practices [6,7]. This study focuses on children and young people with restrictive AN, at high risk of developing RS, and compares RS risk management and clinical outcomes between two initial treatment settings.
RS is defined as severe electrolyte and fluid shifts associated with metabolic abnormalities in malnourished patients undergoing refeeding, whether orally, enterally, or parenterally [1,8]. Its hallmark feature is hypophosphatemia. After prolonged fasting, as seen in AN, reserves of potassium, magnesium and phosphate are already depleted. Processes involved in moving from the fasted to fed state result in a further decrease of serum concentrations of these minerals [9]. Although prevalence of the syndrome in adolescents with AN is low, it is associated with high morbidity and mortality [2,10,11].
Junior MARSIPAN (management of really sick patients under 18 years old with anorexia nervosa), provides national guidance on the management of AN in adolescents in the UK [11]. This guidance originates from the MARSIPAN report, which identified a need amongst clinicians for guidance when managing adults with AN [12]. It sets out clear criteria and thresholds to allow clinicians to assess medical instability and risk of refeeding in patients with AN [11]. These criteria help to differentiate patients requiring hospitilisation for medical stabilisation and refeeding from those that can be safely managed in an outpatient setting. The junior MARSIPAN tool provides a framework for assessing physical risk (Figure 2).
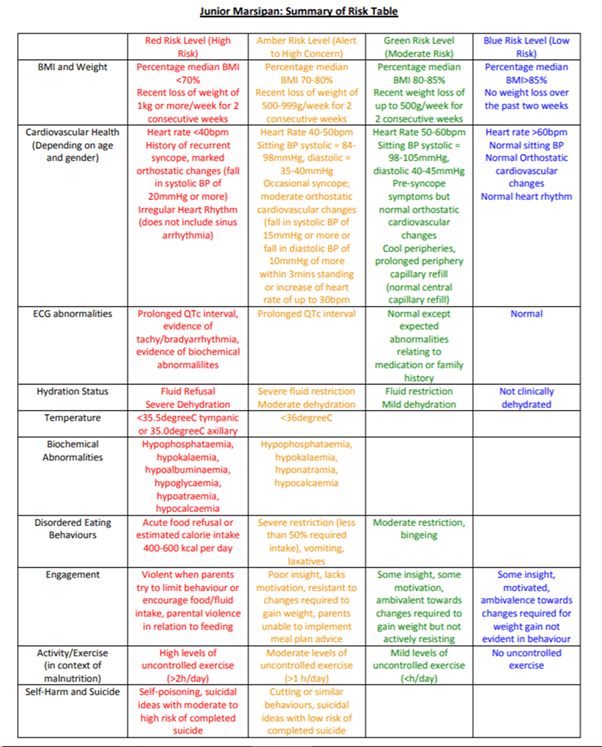
Figure 2: Junior MARSIPAN risk assessment tool.
Disparities in refeeding recommendations worldwide have led to a lack of consensus on how to refeed these individuals safely [7]. Large variations in recommendations on starting energy intakes, prophylactic phosphate supplementation and the use of oral nutritional supplements exist in guidance documents across countries [13-17]. However, an association between the initial degree of malnutrition and development of hypophosphatemia, a predictor of RS, has been widely reported [9,18-20].
A contrast exists between current guidance and emerging evidence on refeeding in AN. The latter encourages a more aggressive strategy for increased remission rates, whilst the former advocates a conservative approach to ensure safety [5,11,20]. Garber and colleagues concluded, in their 2018 systematic review, that initial higher calorie feeding is feasible in moderately malnourished patients with AN, and that although higher calorie approaches to refeeding appear safe in combination with close medical monitoring, there is insufficient evidence to support changes in current standards in severely malnourished patients [3].
Although a lack of intervention studies has previously hindered advancements in refeeding practices in this group, a recent study has become the first randomised controlled trial (RCT) in this area. O’Connor and colleagues, found in 2016 that higher energy starting rates were associated with greater weight initial gain, without increased incidence of RS, suggesting that higher calorie feeding may be safe and preferable [4].
Further studies are needed to define risk factors related to the development of the refeeding syndrome specific to this group of chronically malnourished and underweight individuals. Whilst the evidence base supporting higher energy initial feeding continues to grow, national guidance remains the same. Further research on refeeding practices in adolescent AN is essential to support a review of current guidance and to optimise RS management and treatment outcomes in this group.
The overall aim of this piece of work was to compare refeeding management, nutritional and clinical outcomes in young people with AN admitted initially to inpatient care (before commencing outpatient treatment) with those managed solely in outpatient care. The hypothesis that higher calorie initial feeding (1500 kcal/d), in the absence of prophylactic oral phosphate supplementation or incremental increases, does not increase incidence of RS complications, compared with lower energy intakes (1200 kcal) with phosphate supplementation or stepped increases, was tested.
Data collection
Ethical approval was sought from the Audit Project Manager for the CAMHS CAG in the South London and Maudsley Trust. Ethical approval was also granted by London Metropolitan University ethics committee.
MCCAED research group were consulted to gain access to the electronic data base containing information on patients referred to the service over the past 5 years. The research group compiled data on all patients referred to MCCAED, served by the South London and Maudsley NHS Trust, who were treated between January 2015 and December 2019. The Junior MARSIPAN checklist, a tool used to measure medical instability in patients with anorexia nervosa, alongside consultation with a consultant paediatrician specialising in eating disorders, was used to create a data capture tool.
Electronic patient records were searched and all cases with a diagnosis of anorexia nervosa, and were assessed for eligibility, cases with atypical anorexia nervosa or other eating disorder diagnoses were excluded at this point. Baseline data (Table 1), documented in initial assessment reports completed by the assessing team of clinicians, was collected for all cases. Cases missing essential data at baseline were excluded. Cases from outside the local area served by the trust, that were not accepted for treatment and who were still receiving treatment, were excluded at this point.
| Characteristic | Baseline | Time 1 | Time 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Inpatients (n=19) | Outpatients (n=40) | P value | Inpatients (n=19) | Outpatients (n = 40) | P value | Inpatients (n = 19) | Outpatients (n = 40) | P value | |
| Age, years | 13.6 ± 1.5 | 14.7 ± 1.8 | 0.03 | - | - | - | - | - | - |
| Weight, kg | 39.4 ± 7.8 | 42.5 ± 7.9 | 0.17 | 39.4 ± 7.8 | 42.7 ± 7.9 | 0.15 | 41.5 ± 8.3 | 43.7 ± 7.7 | 0.32 |
| Height, cm | 161.2 ± 9.6 | 162.9 ± 8.4 | 0.49 | - | - | - | - | - | - |
| BMI, kg/m2 | 15.1 ± 1.8 | 15.9 ± 2.2 | 0.18 | 15.1 ± 1.8 | 15.9 ± 2.2 | 0.18 | 15.9 ± 1.6 | 16.5 ± 2.1 | 0.37 |
| mBMI, % | 78.1 ± 9.1 | 79.9 ± 10.3 | 0.53 | 78 ± 9.1 | 81 ± 11 | 0.3 | 81.6 ± 8.5 | 82.3 ± 9.7 | 0.62 |
| Weight loss, kg | 0.86 ± 0.7 | 0.38 ± 0.7 | 0.003 | - | - | - | - | - | - |
| Intake, kcal/d | 374 ± 205 | 621 ± 348 | 0.001 | 1331 ± 309 | 1500 ± 0 | <0.001 | 2321 ± 322 | 2437 ± 202 | 0.09 |
| Temperature, °C | 36.5 ± 0.7 | 36.5 ± 0.5 | 0.56 | 36.4 ± 0.2 | 36.5 ± 0.2 | 0.94 | 36.5 ± 0.1 | 36.4 ± 0.4 | 0.23 |
| QTc, ms | 396 ± 25 | 397 ± 20 | 0.19 | 407 ± 9 | 405 ± 30 | 0.89 | 400 ± 0 | 389 ± 3 | 0.33 |
| Heart rate, bpm | 66 ±24 | 60 ± 18 | 0.17 | 57 ± 12 | 59 ± 12 | 0.63 | 66 ± 14 | 55 ± 12 | 0.04 |
| Glucose, mmol/L | 4.4 ± 1.1 | 4.6 ± 1.2 | 0.57 | 4.6 ± 1.2 | 3.3 ± 0.7 | 0.05 | 4.2 ± 0.4 | 3.2 ± 1.7 | 0.27 |
| Phosphate, mmol/L | 1.1 ± 0.3 | 1.2 ± 0.2 | 0.01 | 1.2 ± 0.2 | 1.3 ± 0.1 | 0.02 | 1.4 ± 0.2 | 1.3 ± 0.2 | 0.14 |
| Magnesium, mmol/L | 0.87 ± 0.1 | 0.93 ± 0.1 | 0.001 | 0.82 ± 0.1 | 0.89 ± 0.1 | 0.002 | 0.82 ± 0.1 | 0.9 ± 0.1 | 0.001 |
| Potassium, mmol/L | 3.9 ± 0.4 | 4.2 ± 0.3 | 0.04 | 3.8 ± 0.5 | 4.3 ± 0.4 | <0.001 | 4.1 ± 0.4 | 4.4 ± 0.4 | 0.002 |
| Calcium, mmol/l | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.02 | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.26 | 2.2 ± 0.1 | 2.2 ± 0.1 | 0.52 |
| WCC, 109/L | 4.9 ± 1.5 | 5 ± 1.1 | 0.82 | 4.3 ± 1.6 | 5.2 ± 1.4 | 0.02 | 4.7 ±1.3 | 5.2 ± 1.4 | 0.34 |
| Neutrophils, 109/L | 2.7 ± 1.4 | 2.7 ± 0.9 | 0.33 | 2.1 ± 1 | 2.5 ± 1 | 0.09 | 2.4 ± 0.8 | 2.6 ± 1.1 | 0.49 |
| AST, units/L | 22 ± 4 | 27 ± 8 | 0.04 | 26 ± 18 | 25 ± 9 | 0.3 | 23 ± 12 | 26 ± 7 | 0.42 |
| GGT, units/L | 9 ± 4 | 14 ± 7 | 0.004 | 9 ± 3 | 13 ± 7 | 0.06 | 9 ± 5 | 13 ± 7 | 0.06 |
| Note: Data are mean ± SD. P values represent comparisons between inpatient and outpatient groups obtained from independent-sample t test and Mann-Whitney u test. BMI, body mass index; mBMI, median BMI for height and gender; WCC, white cell count; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase. P<0.05 indicates significance. | |||||||||
Table 1: Comparison between inpatient and outpatient groups for cardiovascular, anthropometric and nutrition variables at baseline, time 1 and time 2.
| Characteristic | Baseline | Time 1 | Time 2 | ||||||
| t/Z* value | 95% CI | P value | t/Z* value | 95% CI | P value | t/Z* value | 95% CI | P value | |
| Age, years | -2.124* | - | 0.03 | - | - | - | - | - | - |
| Weight, kg | -1.139 | -7.46, 1.34 | 0.17 | -1.456 | -7.86, 1.26 | 0.15 | -1 | -6.69, 2.24 | 0.32 |
| Height, cm | -0.696 | -6.61, 3.2 | 0.49 | - | - | - | - | - | - |
| BMI, kg/m2 | -1.357 | -1.92, 0.37 | 0.18 | -1.373 | -2.03, 0.38 | 0.18 | -0.900* | - | 0.37 |
| mBMI, % | -0.626 | -7.25, 3.79 | 0.53 | -1.041 | -9.1, 2.89 | 0.3 | -0.502* | - | 0.62 |
| Weight loss, kg | -2.921* | - | 0.003 | - | - | - | - | - | - |
| Intake, kcal/d | -2.921* | - | 0.001 | -5.577* | - | <0.001 | -1.674* | - | 0.09 |
| Temperature, ⁰C | -0.595* | - | 0.56 | -0.068* | - | 0.94 | -1.522* | - | 0.23 |
| QTc, ms | -0.858* | - | 0.19 | 0.149 | -27.7, 31.5 | 0.89 | -1.633* | - | 0.33 |
| Heart rate, bpm | -0.958* | - | 0.17 | -0.489 | -9.64, 5.89 | 0.63 | 2.224 | 0.83, 22.2 | 0.04 |
| Glucose, mmol/L | -0.568 | -1.09, 0.61 | 0.57 | -4.887* | - | 0.05 | 1.14 | -0.83, 2.78 | 0.27 |
| Phosphate, mmol/L | -2.262* | - | 0.01 | -2.524 | -0.23,-0.03 | 0.02 | 1.507 | -0.03, 0.20 | 0.14 |
| Magnesium, mmol/L | -3.387 | -0.09,-0.03 | 0.001 | -3.326 | -0.1, -0.03 | 0.002 | -3.754 | -0.12, -0.04 | 0.001 |
| Potassium, mmol/L | -2.166 | -0.39, -0.02 | 0.04 | -4.589 | -0.75,-0.29 | <0.001 | -3.285 | -0.60, -0.14 | 0.002 |
| Calcium, mmol/l | -2.312 | -0.08, -0.01 | 0.02 | -1.129 | -0.05, 0.01 | 0.26 | 0.648 | -0.03, 0.05 | 0.52 |
| WCC, 109/L | -0.234 | -0.78, 0.62 | 0.82 | -2.015* | - | 0.02 | -0.96 | -1.36, 0.49 | 0.34 |
| Neutrophils, 109/L | -0.455* | - | 0.33 | -1.375* | - | 0.09 | -0.689 | -0.86, 0.43 | 0.49 |
| AST, units/L | -1.818* | - | 0.04 | -0.534* | - | 0.3 | -0.815 | -8.52, 3.64 | 0.42 |
| GGT, units/L | -2.637* | - | 0.004 | -1.595* | - | 0.06 | -1.899* | - | 0.06 |
| Note: *P values represent significance of comparisons between inpatient and outpatient groups obtained from independent-sample t test and Mann-Whitney u test. CI, confidence interval. BMI, body mass index; mBMI, median BMI for height and gender; WCC, white cell count; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase. P<0.05 indicates significance. | |||||||||
Table 2: Comparison between inpatient and outpatient groups for cardiovascular, anthropometric and nutrition variables at baseline, time 1 and time 2; test statistics, confidence intervals and significance values.
Every patient presenting to the MCCAED service received a medical examination by a specialist doctor at assessment. The junior MARSIPAN checklist, in combination with clinical expertise, was used by the assessing clinician to assign a risk factor to each case to identify those cases that were most medically unstable and at highest risk of developing the refeeding syndrome. Hospitalisation for medical stabilisation after assessment, prior to returning to outpatient treatment, was based on a combination of clinical judgement and physical risk guided by the Junior MARSIPAN. This was not based on an objective measure of risk, but rather the holistic assessment of multiple medical and psychological parameters and clinical expertise.
Only cases documented by the assessing doctor, as high risk of physical deterioration and of developing refeeding syndrome were included in the final statistical analysis. Cases that remained in outpatient care for treatment at MCCAED, and those transferred to inpatient care at Kings College Hospital for medical stabilisation for up to one week prior to receiving outpatient treatment at MCCAED, were included. Two groups were created based on initial location of care, outpatients, and inpatients. All inpatient cases were transferred back to outpatient care once medically stable, after a maximum period of one week.
Data was collected at three time points, baseline, time 1 and time 2. Baseline described data collected from initial assessment reports for all cases on first contact with the MCCAED service. Time 1 described data collected for all cases after commencement of their prescribed, refeeding meal plan. For the inpatient group this was 1200 kcal on day 1, with increments of 260 kcal per day for 5 days, and for the outpatient group this was 1500 kcal per day (from day 1) without any increments until transfer to full weight restoration meal plan, typically after one week. Time 1 for the outpatient group was between days 3-5 (exact day was determined by when their second set of blood results were available) and for the inpatient group this was between days 2 and 4 (similarly, exact day was determined by their blood test results). Time 2 described data collected for all cases on prescription of their final weight restoration meal plan, this ranged from 2000-2500 kcal per day for all cases across both groups. For the inpatient group this was day 6, and for the outpatient group this was between days 7 and 10 (exact day was determined by their next scheduled appointment with the MCCAED service). For both groups, bloods tests were repeated at time 2.
Baseline, time 1 and time 2 data collected included age, gender, weight, height, percentage median body mass index, actual body mass index, weight loss per week for 1 month prior to assessment, daily energy intake, temperature, heart rate, QT interval, blood glucose, phosphate, magnesium, potassium, corrected calcium, white blood cell, neutrophil, aspartate aminotransferase (AST) and gamma-glutamyl transpeptidase levels (GGT), as well as initial treatment setting, inpatient or outpatient.
Baseline weight loss was determined based on the information collected routinely during the initial assessment. Weight history was collected for all cases. Average weight loss per week was calculated from the overall weight loss reported in the month leading up to assessment. Baseline daily energy intake was determined based on a comprehensive diet history collected routinely at every initial assessment. Total daily energy intake was reported by the assessing clinician within the initial assessment report in most cases. For those without, diet information was added to nutritional analysis software to calculate total daily energy.
Study design
This study was a retrospective case note review of electronic patient records that was undertaken to explore characteristics of patients treated under the MCCAED team over the past 5 years over the initial refeeding period. The study included young people between the ages of 10-18, in treatment with MCCAED for anorexia nervosa according to the DSM5 diagnostic criteria. Cases were assigned to 2 independent groups, inpatient and outpatient, based on their initial treatment setting during the first week of treatment. These groups were compared using statistical analysis to explore differences and similarities in their characteristics and their outcome measures.
Selection of participants
Children and adolescents diagnosed with restrictive anorexia nervosa and accepted for treatment by MCCAED between January 2015 and December 2019 were included in the study. To ensure that groups were comparable in terms of risk of developing refeeding syndrome, only cases documented as at high physical risk of deterioration by the assessing clinician were included for both groups.
Statistical analysis
Baseline, time 1 and time 2 data was compiled, screened for missing variables and added to the IBM SPSS 26 software program for statistical analysis. Descriptive statistics were used to explore mean characteristics of each group for baseline, time 1 and time 2 data.
Data were tested for normality of distribution using the Shapiro-Wilk test, due to small sample size. Levene’s test for homogeneity of variance was used to identify if normally distributed variables had equal variance across groups. Independent samples t-tests were used to compare mean differences between groups for those normally distributed variables, whilst the Mann Whitney U test compared variables that did not follow normal distribution. Paired sample t-test and the Wilcoxon signed-rank test were used to compare differences within groups. Significance was determined by a p value, corrected for all ties, of less than 0.05 for all tests.
Case selection
Selection of participants for inclusion in the study is displayed in Figure 1 (n=59). Cases were excluded based on missing key data (n=106), not meeting inclusion criteria (n=39) and not being high risk (n=122). Reasons for not meeting inclusion criteria included, lacking a clinical diagnosis of AN on assessment by MCCAED, not being accepted for treatment or not attending treatment, not residing in the local are or moving during treatment to another service and having a physical health comorbidity that affected treatment. High risk was based on medical risk assigned by the assessing doctor and documented on the assessment report. Two groups were created and included in final analysis: an inpatient and outpatient group (n=19 and n=40).
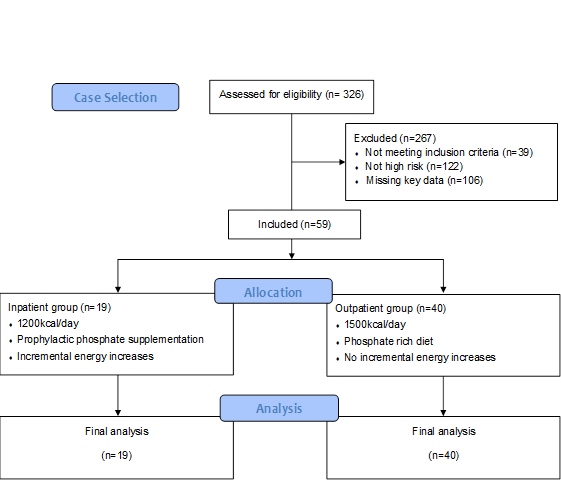
Figure 1: Flow chart of participants included in the current study.
Baseline characteristics of groups
Significant differences existed between the inpatient and outpatient groups at baseline (Table 1). Mean age and energy intake were lower amongst the inpatient group, whilst mean weight loss was higher in this group. Baseline blood results: phosphate, magnesium, potassium, calcium, AST and GGT in the inpatient group were significantly lower than those of the outpatient group. However, all blood markers were within reference ranges for both groups (Tables 1 and 2).
Time 1 characteristic of groups
Significant differences were found between blood markers (potassium, phosphate, magnesium and white cell count) and mean energy intake between groups, with all of these variables being significantly lower amongst the inpatient group when compared with outpatients (Tables 1 and 2).
Time 2 characteristics of groups
Mean heart rate was significantly lower amongst the outpatient group compared to the inpatient group. Mean blood results: potassium and magnesium were significantly lower in inpatients compared with outpatients (Tables 1 and 2).
Summary of results
This study represents the 5-year experience of refeeding children and young people in a large national eating disorders service. In line with other studies, no cases of the clinical refeeding syndrome were found. In contrast to other UK centres, initial refeeding calories for those managed in outpatients are higher than national guidance (1500 kcal vs 1200 kcal). This group of high risk adolescents with restrictive anorexia nervosa were found to display minimal to no signs of RS symptoms upon refeeding regardless of refeeding protocol used in either setting.
Hospital admissions were more likely in adolescents with lower energy intakes and higher rates of weight loss at baseline, though without derangements in other markers of physical health recorded by the Junior MARSIPAN tool. Characteristics of inpatients and outpatients were comparable at all-time points and although various biochemical markers were lower in the inpatient group; these markers were generally not outside of the clinical reference ranges.
This study identified that protocols used in the management of refeeding risk in either setting were not associated with adverse effects in either group observed within the study period. Clinical experience and judgement were often used in combination with the current guidance in refeeding risk management.
Differences in characteristics of groups
Physical health assessments were used to measure medical instability in this study. National guidance, as well as local guidance, advises that admissions to inpatient care may be required for medical stabilisation and refeeding for those at highest risk [11,21]. However, hospitalisation in this study was not based on immediate medical risk but rather the rate of physical deterioration, determined by rate of weight loss and daily energy intake at assessment. Those admitted to inpatient care had significantly lower energy intakes and significantly higher rates of weight loss when compared with cases who remained in outpatient care.
These findings are consistent with recent literature on the medical management of AN, attributing high physical risk predominantly to rapid weight loss and malnutrition [22,23]. Although sample size of cases included in final analysis was small, this was representative of the number of high risk adolescents treated by a large specialist centre for eating disorders over a five year period, and may be indicative of the proportion of this population that are at highest physical risk [24].
Limitations of the study
The main weakness of the study, like most retrospective reports, was the large volume of missing data, resulting in many cases being excluded. Other limitations were the small sample size and difference in group sizes. Although the inpatient cohort was representative of admission rates in the MCCAED service, the outpatient cohort was significantly reduced by exclusions from missing data. Specific statistical methods were chosen based on these limitations to ensure that results were still valid. Further testing could be carried out to include previously excluded cases to determine if results differ.
A confounding factor of this study was the prophylactic supplementation of phosphate within the inpatient group. All inpatient cases received oral phosphate supplementation. Although cases remaining as outpatients were prescribed a phosphate rich diet, they did not receive supplementation. Serum phosphate levels in the inpatient group may have been influenced by this supplementation and limited the number of cases that developed hypophosphatemia. However, both groups were shown to be comparable in terms of medical instability and at similar risk of developing refeeding hypophosphatemia. The fact that no patients in the outpatient group developed hypophosphatemia supports the theory that phosphate supplementation is not required to prevent this complication.
In conclusion, results from this five year retrospective case note review support the hypothesis that refeeding adolescents with AN, at high risk of developing RS, with higher energy feeds than that advised by national guidance, in the absence of prophylactic phosphate supplementation or incremental energy increases, does not increase the risk of developing refeeding complications, such as hypophosphatemia. These findings support recent evidence that advocates a less conservative refeeding approach and a review of current national guidance.
Conclusions drawn from the results of this study supported five recommendations
1. All adolescents diagnosed with AN require a full physical health assessment at baseline that can be used as an objective measure of medical instability. 2. Higher calorie initial feeds of 1500 kcal/d should be considered for most cases (those >60% mBMI), regardless of treatment setting. Regular review and monitoring of biochemistry and physical observations should be carried out to ensure safety of higher calorie feeding. 3. Phosphate should be prescribed only if medically indicated for certain cases, rather than universally administered as a prophylactic intervention in inpatient care. A diet rich in phosphate should be advised on during the refeeding period for all cases. 4. National guidance on refeeding management in adolescent AN should be reviewed and updated to include current evidence to advance practice in this area, with rapid weight loss likely being a better indicator of hospitalisation than other markers.
Findings from this study, similar to those of other recent literature in this area, support a move towards less conservative refeeding in adolescent AN. These results provide additional evidence to suggest that medical instability should not be assumed in all low weight, malnourished adolescents, but should be objectively assessed and managed on a case by case basis. A greater understanding of the physiological response to refeeding of adolescents with AN is essential to improve and update current guidance.
Ethical approval was sought from the Audit Project Manager for the CAMHS CAG in the South London and Maudsley Trust. Ethical approval was also granted by London Metropolitan University ethics committee.
Not applicable
The datasets supporting the conclusions of this article are available in the ‘Google Drive’ repository, https://drive.google.com/ drive/u/1/folders/1Zv_zMh3a1llQY2AfYzH8xQ6MqGd9Fc4R.
The authors declare that they have no competing interests.
There are no funding sources to declare.
CB was responsible for study design, data collection and interpretation of results as well as drawing of conclusions. SC was involved in study design, advising on medical aspects of study and creation of data collection tool. SI was responsible for providing supervision for all elements of the project.
Thank you to Mima Simic, Catherine Stewart, Anna Konstantellou and James Davis for their support and advice in carrying out this piece of work.
Citation: Brennan C, Chapman S, Illingworth S (2021) A Retrospective Analysis of Refeeding Outcomes Over 5-years in a Specialist Adolescent Eating Disorder Centre in the UK. J Nutr Food Sci. 11:813.
Received: 09-Jul-2021 Accepted: 23-Jul-2021 Published: 30-Jul-2021
Copyright: © 2021 Brennan C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.