Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Background: Diabetes is a multifactorial, heterogeneous metabolic disorder with micro and macro vascular complications that results in noteworthy concerns in human life and its treatment has also become more challenging due to the adverse effect of current medicines. In this scenario, the use of dietary food supplements is most advocated, because it’s mainly derive from medicinal plants, fruits, vegetables and grains.
Objective: The present study aimed to investigate the effect of novel herbal formulation (Glymin sprinkle) enriched with multigrain inhibits oxidative stress, AGE formation and pancreatic pathology associated with diabetes.
Methods: Herein, we report the effect of novel herbal formulation [Glymin sprinkle (GS)] enriched with multigrain on Streptozotocin induced diabetic model. Diabetes was induced by a single dose of Streptozotocin (60 mg/kg b.wt) injection through intra-peritoneum. Efficacy of GS was determined by analysing various parameter like antioxidants status, liver enzyme markers, glycation of haemoglobin, blood glucose level, glyoxalase-1 protein expression, histological analysis were analysed.
Results: From the results, it strongly supported that GS significantly enhance the antioxidant status, glyoxalase 1 expression and maintain the normal structure of pancreas. Also, it helped to reduce the overwhelmed blood glucose level, liver enzymes and glycated haemoglobin.
Conclusion: All these results suggested that GS has potent anti-diabetic effect. Thus, it can be recommended to diabetic patients.
Diabetes mellitus; Glymin sprinkle; Antioxidants; Glyoxalase-1; Liver enzymes
Diabetes mellitus is a chronic metabolic disease which is characterised by hyperglycemia due to insulin deficiency or resistance. There is an inter-relation between the genetic and environmental factors in contributing β-cell dysfunction [1]. Studies showed that, life style changes and unhealthy food pattern are the basic reasons for the upsurge in diabetes. As its prevalence is outstripping in the recent years, diabetes is tagged as the disease of 21st century [2]. According to the survey in 2017, there are 451 Million people suffering from diabetes and its related complications all over the world. As per this survey, the prevalence of diabetic patients will rapidly increase and it turns around 693 million in 2045 [3]. The exact mode of pathogenesis of diabetes is still unknown, but the studies have been reported that oxidative stress plays a crucial role in the pathogenesis of diabetes. Normally, the reactive oxygen species (ROS) generated during a stressed condition will be scavenged by antioxidants present in our body but in the case of diabetic condition there will be an excessive production of free radicals, so that the antioxidant activity will be diminished considerably. Thus, it can lead to the severe damage of macromolecules and the formation of advanced glycation end product (AGE) [4]. AGE is a complex heterogeneous compound formed via non-enzymatic glycation of biomolecules. It will trigger the activation of intracellular pathways especially, NF-kB pathway which is responsible for the expression of various pro-inflammatory cytokines, monocyte chemoattractant peptide 1 as well as receptor of AGE (RAGE). Recent evidence showed that AGEs is one of the major contributing factor leading to insulin resistance and causing damage to the cells [5]. As a result of all these changes, it will lead to the development of diabetic associated diseases such as nephropathy, retinopathy, neuropathy, coronary artery problems etc., [6].
Treatment of diabetes mainly include oral hypoglycemic drugs such as glibenclimide, metformin, acarbose etc and insulin injection.
Normally, these antidiabetic drugs are prescribed to consume for a long term. In fact, it can lower the blood glucose level but prolonged usage will cause lot of side effects which can ultimately lead to organ damage [7]. So, the treatment of diabetes has become a major challenge faced by the medical experts. Since from the ancient time, herbal medicines are effectively used in the treatment of various diseases but the establishment of modern medicine positioned it in the mainstream of medical field for the treatment of vast number of diseases. Nowadays, the trend has changed and traditional herbal medicine regain the preference of the people as their primary health care remedy [8].There are so many natural products which are capable of managing diabetes by reducing the blood glucose, enhancing the antioxidants and keeping the cells healthier. Many research studies have been showed that polyherbal formulations are very efficacious in controlling the diabetes and diabetic associated changes in the body [9]. Thus, researchers are exploring the scope of pharmaceutical properties of combination of various natural and herbal products such as plant parts or fruits, cereals, spices etc.
Jackfruit (Artocarpus heterophyllus) is one such interesting fruit which seek the attention of people only because of its medicinal value in the recent time. It is a tropical fruit comes under the family Moraceae and native to India, Indonesia, some part of South-East Asia, Australia, Florida, Brazil. It consists of rich dietary fibre, carbohydrates, vitamins, minerals and have only low caloric content. It is used in the treatment of various diseases as it possesses different pharmacological activities such as anti-diabetic, anti-inflammatory, antioxidant, anti-microbial activities [10]. Even though it has such a wide range of health benefits, jackfruit is still underutilized. The other source is cereals especially the grains which is considered as a part of healthy diet and it contain some unique phytochemicals and other components to provide its nutritional and pharmacological value. Furthermore, cereals have the ability to improve the challenges tied up with the diabetes such as glucose intolerance, insulin resistance, oxidative stress etc. Thus, cereals are recommended as a potent antidiabetic agent [11]. By considering all these facts, the present study aims to evaluate the anti-diabetic effect of novel herbal formulation enriched multigrain supplement (hereinafter referred to as ‘Glymin sprinkle’) on experimental diabetic model.
Chemicals
High quality analytical grade reagents and chemicals were used for the study. SGOT, SGPT, ALP and glucose analysis kits are purchased from Ray Biotics India Pvt. Ltd. ELISA Kits and antibodies were purchased from Sigma Aldrich USA. Crystal Chem USA kit were used for HbA1C analysis.
Preparation of glymin sprinkle
100 gm of Glymin sprinkle contains Artocarpus heterophyllus- 25.8 g, Cicer arietinum- 25.8 g, Elusine coracana- 10 g, Panicum sumatrense- 10 g, Panicum miliaceum- 10 g, Moringa oleifera- 10 g, Curcuma longa- 3 g, Phyllanthus emblica- 5 g, KAL 10 powder- 3.81 g.
Animals
Adult male Wistar rats (weighing 150 gm) were used for this study. They were kept in a controlled environment, temperature (24- 60°C), humidity (55-60%) and photoperiod (12:12 hr light-dark cycle). A commercial laboratory diet (Amrut Laboratory Animals feeds, Maharashtra, India) and tap water were provided ad libitum.
The animals received humane care, in compliance with the host institutional animal ethics guidelines. All experiments were conducted as per the guidelines of the animal ethics committee (CBLRC/IAEC/10/01-2019) of the host institute.
Experimental design for diabetic inductions
Animals were divided into four groups (n=6 per group).
Group I was treated as Normal control rats (N).
Group II was kept as Diabetic control (DC) by inducing Streptozotocin (60 mg/kg.bwt).
Group III was treated with Standard drug (DC+GB) (Glibenclamide) (5 mg/kg.bwt).
Group IV was treated with Glymin sprinkle (DC+GS) (80 mg/ kg.bwt).
Induction of diabetes
Streptozotocin (60 mg/kg.bwt) was given to all the rats except the normal control group by intraperitoneal injection. Glucose solution was given to the rats after the induction of Streptozotocin. The blood sugar level was measured after 3 days of administration. Rats that have a blood sugar level above 200 mg/dl was selected for this study. GB and GS were administered by intra-gastrically (i.g.) for 21 days. After the experimental duration, rats were sacrificed after overnight fasting by euthanasia. For histological analysis, pancreas tissue was dissected and fixed in 10% buffered formalin and it was then processed and embedded in paraffin. Blood and liver tissue were also collected for various biochemical estimations.
Estimation of blood parameter
Glycated hemoglobin level (HbA1c) was measured in the blood by using HbA1c assay kit, Crystal Chem USA.
Estimation of pancreatic antioxidant enzymes
Estimation of Catalase (CAT): The enzyme activity was assayed by homogenizing tissue in Tris buffer and centrifuged at 40000 rpm. Supernatant was collected and used for the assay. Reaction mixture contained phosphate buffer, 2 mM H2O2 and the enzyme extract. The decrease in the absorbance at 230 nm was measured spectrophotometrically. The enzyme activity was expressed in terms of units per mg protein [12].
Estimation of Superoxide Dismutase (SOD): Tissue was homogenized in Tris EDTA buffer and centrifuged. Assay mixture contained 1.2 ml sodium pyrophosphate buffer, 0.1 ml of phenazine methosulphate, 0.3 ml of nitroblue tetrazolium, 0.2 ml NADH, 0.2 ml of homogenized tissue and water in a total volume of 3 ml. Reaction was started by the addition of 0.2 ml NADH. After incubation at 30°C for 90 seconds, the reaction was stopped by the addition of 1 ml glacial acetic acid. Reaction mixture was stirred vigorously and shaken with 4 ml of n-butanol. The mixture was allowed to stand for 10 min, centrifuged and butanol layer was taken out. The colour intensity of the chromogen in the butanol fraction was measured at 560 nm against butanol as blank. A system devoid of the enzyme served as control. One unit of the enzyme activity is defined as the enzyme concentration required to inhibit the chromogen production by 50% in one minute under the assay conditions. Specific activity is expressed in unit/mg of protein [13].
Estimation of Glutathione Peroxidase (GPx): Tissue homogenate (10%) was prepared in 0.125 M sucrose, centrifuged at 10,000 xg for 30 min and the supernatant fraction was used for the assay. Activity was determined in 2 ml phosphate buffer containing 0.2 ml EDTA, 0.3 ml sodium azide, 0.1 ml of glutathione reduced, 0.1 ml NADPH and 0.5 ml water. To this 0.2 ml of enzyme source was added and the mixture was allowed to incubate at room temperature for 5 min before initiation of the reaction by the addition of 0.2 ml of H2O2 solution. Optical density was measured at 340 nm at 20 second intervals for 3 minutes. Enzyme activity is (defined as μmoles of NADPH oxidized/min/mg protein using 0.25 mM of H2O2 as substrate [14].
Estimation of Reduced Glutathione Content (GSH): Tissue was homogenized in 5 ml precipitating solution. The tube was incubated for 5 minutes at room temperature and then filtered through course grade filter paper. To 0.2 ml filtrate, 3 ml of 0.3 M phosphate solution and 1 ml 0.04% DTNB was added. These tubes were capped, mixed by inversion and contents were read at 412 nm within 4 minutes [15].
Estimation of Thiobarbituric Acid Reactive Substances (TBARS): 1 g tissue was homogenized in 9 volumes of phosphate buffer (0.12 M) pH 7.5, centrifuged and the supernatant was taken. To 0.5 ml of tissue homogenate, 2.5 ml of 10% Trichloroacetic acid (W/V) at pH 3.5 was added. The volume was made up to 3 ml with and then heated in boiling water bath at 95°C for 15 minutes using glass ball as a condenser. Then the solution was centrifuged at 3000 rpm for 10 mins. From that 2 ml of supernatant was transferred to 1 ml of Thiobarbituric acid solution. The tubes were placed in boiling water bath for 15 mins. Once the solution attains room temperature its absorbance at 532 nm was measured against butanol as blank. The lipid peroxidise levels were expressed as mmol of malonaldehyde produced [16].
Estimation of IL-6 and TNF-α level: IL-6 and TNF-α were evaluated using Enzyme linked immunosorbent assay by following the instructions of the manufacturer (Sigma-aldrich, USA).
Western blot analysis for Glyoxalase-1: Briefly, cells were washed twice with Phosphate buffered saline and scraped into a lysis buffer. Protein concentrations were analyzed with a Bio-Rad system. Equal amounts of protein for each sample were electrophoresed through a 10% SDS-PAGE gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes and the blots were subsequently probed with the following Glyoxalase-1 antibodies (1:1000 sigma Aldrich). For detection, horseradish peroxidaseconjugated secondary antibodies were used (1:2000) followed by enhanced chemiluminescence development. Bands were scanned and semi quantified by densitometry. GAPDH was used as an internal control.
Estimation of SGOT, SGPT and ALP: SGOT, SGPT, ALP were measured by using the diagnostic kits from Ray Biotic Company, Pvt. Ltd., India.
Histopathological analysis of pancreas tissue
For the histopathological analysis, pancreas tissue was dissected out and tissue was fixed by dipping into 10% formalin solution. Tissue sections of the pancreas was taken and stained with hematoxylineosin (H&E). For the examination of structural anomalities tissue section was observed under a light microscope and also takes the photographs. Histopathological changes were interpreted by different viewers completely blinded to the experimental protocol.
Statistical analysis
The results were analysed using a statistical program SPSS/PC+, version 11.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was employed for comparison test of significant differences among groups were determined. Pair fed comparisons between the groups was made by Duncan’s multiple range tests. P<0.05 was considered significant.
Effect of GS on blood glucose level
The blood glucose level was monitored periodically and the result showed that the glucose level was increased in the diabetic control group in each respective days as compared to normal. GS treatment gradually decreased the glucose level and restore to the normal level better than the standard drug over the period of 21 days (Figure 1).
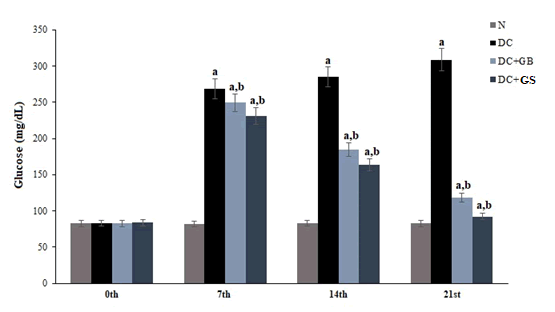
Figure 1: Effect of GS on blood glucose level. Values expressed as average of 6 samples ± SD in each group. ‘a’ -Statistical difference with normal group at P ≤ 0.05. ‘b’ -Statistical difference with diabetic control group at P ≤ 0.05.
Effect of GS in glycated haemoglobin level
In diabetic control group glycation of hemoglobin or HbA1c was significantly higher as compared to the normal group. Supplementation of GS significantly reduced the level of glycated hemoglobin as compared to the standard drug and diabetic control groups. GS treatment brought back the glycated hemoglobin level nearer to normal control (Figure 2).
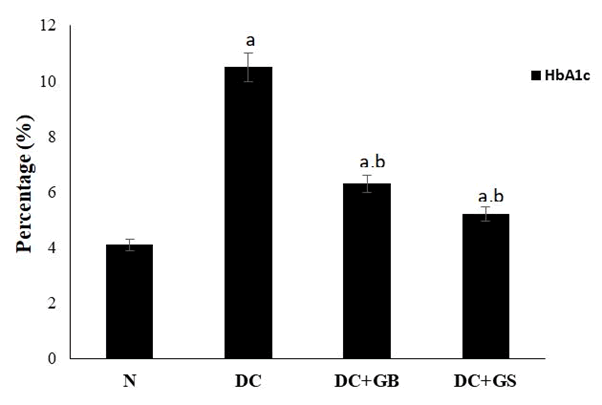
Figure 2: Effects of αLA on the hepatic levels of TNF-α (a) and IL-6 (b) in GalN/LPS-treated rats. Rats were fed a standard AIN-93M diet or an αLA-containing diet for 7 days. After one week from starting the diets, rats were intraperitoneally injected with GalN/LPS or saline. Livers were excised at 10 h after the GalN/LPS injection. Hepatic TNF-α and IL-6 were measured by ELISA. Each value represents the mean ± SEM (n=4-8). The different letters at each measured time represent the statistical differences at p<0.05 among the groups by Tukey-Kramer test or Steel-Dwass test.
Effect of GS on pancreatic antioxidant enzyme
Streptozotocin induction results in the amplified production of free radicals, thus the antioxidant level was significantly reduced in the diabetic control group. But the administration of GS and standard drug considerably enhance the antioxidant status. Moreover, GS significantly improved the level of antioxidants as compared to standard drug and diabetic control as shown in the Figure 3.
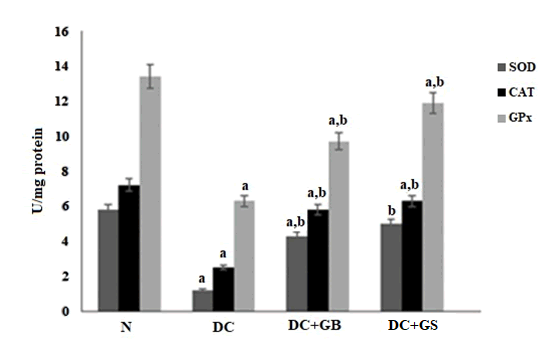
Figure 3: Effect of GS on the activities of antioxidant enzymes. The Values expressed as average of 6 samples ± SD in each group. ‘a’ -Statistical difference with normal group at P ≤ 0.05. ‘b’ -Statistical difference with diabetic control group at P ≤ 0.05.
Effect of GS on GSH level
Comparing the diabetic control group with normal, the nonenzymatic antioxidant GSH was reduced significantly. But the dietary supplementation of GS detoxifies the ROS generated and enhanced the synthesis of GSH as compared the diabetic control (Figure 4).
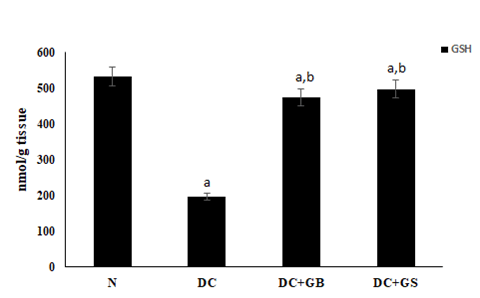
Figure 4: Effect of GS on GSH level. Values expressed as average of 6 samples ± SD in each group. ‘a’ -Statistical difference with normal group at P ≤ 0.05. ‘b’ -Statistical difference with diabetic control group at P ≤ 0.05.
Effect of GS on lipid peroxidation end product
As a result of lipid peroxidation, TBARS level was elevated significantly, which implies the overproduction of free radicals. Scavenging capacity of antioxidants in GS was excellently removed the radicals formed and block the lipid peroxidation as compared to the diabetic control (Figure 5).
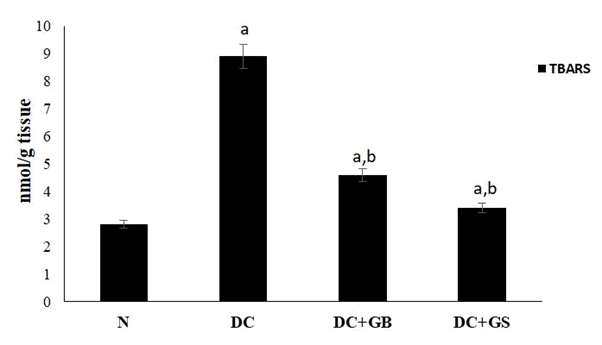
Figure 5: Effect of GS on lipid peroxidation end product level. Values expressed as average of 6 samples ± SD in each group. ‘a’ -Statistical difference with normal group at P ≤ 0.05. ‘b’ -Statistical difference with diabetic control group at P ≤ 0.05.
Effect of GS on liver enzymes
The major marker enzymes SGOT, SGPT, ALP were significantly increased in streptozotocin induced control group compared to normal which clearly shows the liver damage. By the supplementation of GS, it reduced the serum transaminases level almost closer to the normal group (Figure 6).
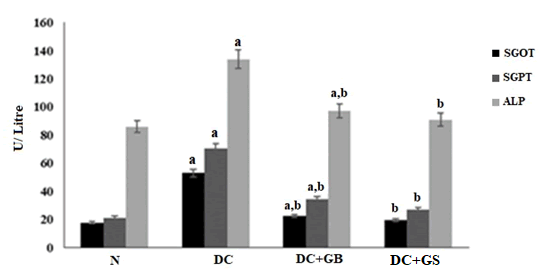
Figure 6: Effect of GS on liver marker enzymes. The values are expressed as mean± SD of six rats in each group. U:SGOT- µmol of oxaloacetate liberated /min/mg protein. U:SGPT- µmol of pyruvate formed /min/mg protein. U:ALP- amount of enzyme to decompose 1 µmol of P-NPP/minute at 25°C. a - Statistical difference with normal control group at P<0.05. b – Statistical difference with diabetic control group at P<0.05.
Effect of GS on the level of Glyoxalase-1
The result showed that the expression of glyoxalase-1 protein is considerably suppressed in the diabetic control group as compared to normal and it was significantly expressed in GS treated rats. Thus, it is clear that glyoxalase system is active to remove the methyl glyoxal developed during diabetes. Results were shown in Figure 7.
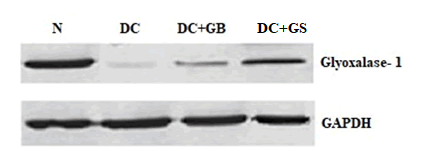
Figure 7: Western blot analysis of Glyoxalase-1. The values are expressed as Mean ± SD for 3 different experiments.
Histopathological analysis of pancreas
The result of histopathology study of pancreas shows in normal rat, the pancreas contains lobules of pyramidal cells with zymogen granules arranged in exocrine serous acinar (EX) units and also normal population of islet of Langerhans (NI). In the case of diabetic control group, the number of islets of Langerhans is reduced due to necrosis (N) and islet atrophy (IA). GB treat group showed mild reduction in number of islet whereas, histology of GS treated group is showed the restoration of islet population similar to the normal (Figure 8).
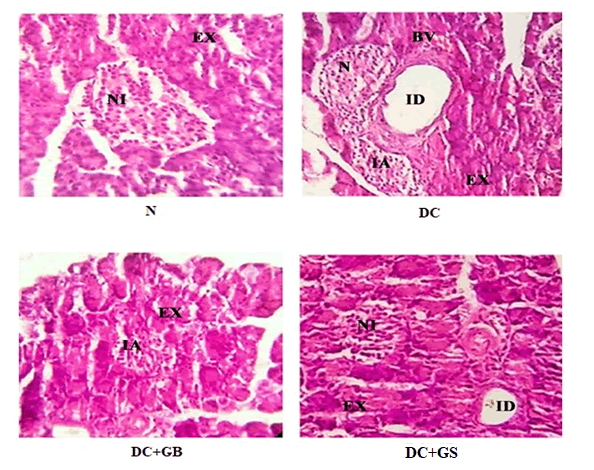
Figure 8: Histopathology of rat pancreas (H&E stain) (40X). Abbreviations- EX- Exocrine pancreas; NI- Normal islet; ID- Interlobular duct; N- Necrosis; IA- Islet atrophy; BV- Blood vessel.
Streptozotocin induced diabetic model is a commonly used experimental model to create a hyperglycemic condition with certain clinical manifestations similar to Type 2 diabetes [17]. Streptozotocin will enter into the pancreatic β-cell through glucose transporter 2 which causes DNA alkylation and trigger the activation of poly (ADP-ribosylation). As a result, nicotinamide adenine dinucleotide (NAD+) and adenosine triphosphate (ATP) will be depleted and an oxidative stress condition will be created that leads to the formation of free radicals resulted in the pancreatic β-cells necrosis [18]. HbA1c is considered as the best marker of diabetes and also it gives the accurate indication of glycemic control. Increased level of HbA1c is a sign of hyperglycemia and also it can lead to cardiovascular diseases and stroke in diabetic patients [19]. Glycated hemoglobin has a correlation with blood glucose level because its formation is depending upon the concentration of glucose in the body [20]. HbA1c is formed by the non-enzymatic reaction of glucose with a free amino group of hemoglobin. From our study, the results showed that diabetic control rats had significantly higher level of glucose, so that it leads to the glycation of hemoglobin and thereby increase the level of HbA1c. GS Treatment significantly reduced blood glucose which means it help to improve the glucose metabolism and inhibits the glycation of haemoglobin.
As a result of hyperglycemia, the normal functioning of pancreatic beta cells will be distressed that leads to a stressed condition due to the formation of more and more ROS and RNS. Various peroxidative reactions will also take place to damage the equilibria of cellular activities. Finally, the outcome will be reduced antioxidant status [21]. SOD, CAT, GPx are the major scavengers of radicals, which act as first line defense against them. GSH is another non enzymatic antioxidant, which also plays significant role in the protection of cells from oxidative stress [22]. Normally, there will be a sudden action of these enzymes during the time of a stressful condition and helps to neutralize the radicals to harmless molecules. In contrast, during diabetic condition this was found to be reduced significantly [23]. In our study, it was found that, in diabetic control group the antioxidant level was compromised due to the high blood glucose associated oxidative stress but supplementation of GS replenishes the antioxidant level, thereby increased the level of SOD, CAT, GPx and GSH as a result reduced the stress condition.
Reduced antioxidants and increased free radicals will lead to the formation of glycated proteins which are responsible for the initiation of lipid peroxidation that damages the normal architecture of cells [24]. Glycated proteins produced due to hyperglycemia will themselves act as free radicals and it will overwhelm during severe diabetes. This is also a reason for causing increased lipid peroxidation [25]. TBARS are the end product of lipid peroxidation and it is also considered as a marker for measuring it [26]. Treatment with GS significantly protects against the formation of lipid peroxides. Altogether, these results clearly suggest that GS possesses strong antioxidant property. Therefore, it can control diabetes by obstructing oxidative stress.
Lipid peroxidation in the liver causes hepatic damage which leads to the leakage of liver enzymes like SGOT, SGPT, ALP into the bloodstream. As a result, there will be an elevated level of liver enzymes in the serum which implies severe liver damage [27]. Our result also revealed that the diabetic control group showed a significantly higher level of liver marker enzymes, but in GS treated group, it helps to protect the hepatocytes from damaging and thus, maintain the normal level liver enzymes.
Methylglyoxal (MG) is a main cause of diabetes, which is the precursor of advanced glycation end product (AGE). MG is highly reactive and its concentration will increase when its precursor like higher glucose increases [28]. It will also influence the magnitude of oxidative stress. Normally, it is controlled by the glyoxalase system in which glyoxalase 1 will initiate the process. Therefore, it is considered as the key regulator of MG level and MG derived AGEs both intracellular and extracellular level. In diabetic patients MG level will amplify up to fourfold and more AGE products will form due to the downregulation of glyoxalase 1 [29]. This is also reflexing in our study, the diabetic control rats showed only slight expression of glyoxalase 1 protein, but GS treated rats upregulated the expression of this protein. That is glyoxalase system was active in GS supplementation group.
In major types of Diabetes, the pancreatic β cells will severely affect along with insulin secretion. Maintaining the β cells alive is very important to keep the glucose level in a controlled manner to reduce the risk of diabetes [30]. Histopathological analysis of pancreas clearly showed the anti-diabetic effect of GS. Due to the overproduction of free radicals, insulin producing beta cell containing island called islet of Langerhans got damaged and steep decrease in the number of cells was observed in diabetic rats. As GS possesses a good antioxidant capacity, this helps them to protect the pancreatic islets thereby we observed a structural improvement of cells.
From the observations, it is clear that novel herbal formulation enriched with multigrain referred as ‘Glymin sprinkle’ can control diabetes significantly by modulating the blood glucose level, HbA1c level, liver enzyme markers, oxidative stress, enhance the glyoxalase level and maintains the normal histology of pancreas Thus, it can be used as a promising anti-diabetic dietary food supplement.
We express our sincere gratitude to Kerala Ayurveda Ltd, Kerala, India for the financial support.
The experiments were conducted as per the guidelines of the animal ethics committee and was approved by the committee with number (CBLRC/IAEC/10/01-2019).
All the authors are declaring the conflict of interest. Glymin sprinkle is a product of Kerala Ayurveda Ltd.
Citation: Sheethal S, Ratheesh M, Jose SP, Asha S, Sandya S, Rajan S, et al. (2021) A Novel Herbal Formulation (Glymin Sprinkle) Enriched with Multigrain Inhibits Oxidative Stress, AGE Formation and Pancreatic Pathology Associated with Diabetes. J Nutr Food Sci. 11:823.
Received: 17-Sep-2021 Accepted: 01-Oct-2021 Published: 08-Oct-2021 , DOI: 10.35248/2155-9600.21.s8.1000823
Copyright: © 2021 Sheethal S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Sources of funding : We express our sincere gratitude to Kerala Ayurveda Ltd, Kerala, India for the financial support.