Journal of Nutrition & Food Sciences
Open Access
ISSN: 2155-9600
ISSN: 2155-9600
Research Article - (2021)
Various parts of Ocimum sanctum Linn. Such as leaves, seeds, roots, fruits, barks, flowers etc., were widely used in traditional medicine to cure a wide range of diseases over the decades. Although several studies include phytochemical screening, antibacterial, antifungal and hyperglycemic activity of Ocimum sanctum. Some parts of Ocimum sanctum remain to be elucidated. The present study includes nutritional analysis, antibacterial activity, antifungal activity and antidiabetic effect of Ocimum sanctum leaves and flowers. From nutritional investigation, the proximate composition moisture, ash, lipid, total sugar, crude fiber, starch, total phenols, vitamin C, vitamin B1 and vitamin B2 contents of leaves were 84.78%, 12.72%, 0.5%, 60%, 14.8%, 13.90%, 0.37%, 14%, 0.48%, 0.24% respectively. On the other hand, moisture, ash, lipid, total sugar, crude fiber, starch, total phenols, vitamin C, vitamin B1, and vitamin B2 contents of flowers were 71.56%, 25.56%, 0.02%, 77%, 5.38%, 16.10%, 0.25%, 42%, 0.27%, 0.33%, respectively. The leaves and flowers also contain significant amounts of Ca, Zn, P, and iron. Ocimum sanctum showed activity against both gram negative bacteria and pathogenic fungi. Leaves and stem extracts showed antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Sarcina lutea, Pseudomonas aeruginosa. These extracts also showed antifungal activity against Aspergillus niger, Candida albicans and Saccharomyces cerevisiae. Both leaves and flowers showed significant weight gains in Steptozotocin-induced diabetic rats compared to vehicle control rats. Finally, the extract treatment remarkably reduced blood glucose level in diabetic rats compared to vehicle control rats. Therefore, Ocimum sanctum could be considered as an effective and alternative treatment for bacterial, fungal infections and diabetes.
Ocimum sanctum; Nutrition; Antimicrobial; STZ-diabetic rats; Anti-diabetics
Ocimum sanctum Linn is an aromatic herb in the family Lamiaceae (Lamiales) [1]. Ocimum sanctum is a small medicinal herb locally known as “TULSI” is found throughout Bangladesh and many other countries in the world [2]. It has been traditionally used for thousand years in Ayurvedic medicine for its many healing properties [3]. Daily intake of Ocimum sanctum in the diet is safe for human use and can prevent and improve various health conditions [4]. The leaves and flowers of the plant are the major parts that may contain many active components. The nutritional and pharmacological properties of the whole herb in its natural form, as it has been traditionally used, may results from synergistic interactions of many different active phytochemicals [3]. Advanced research has revealed potential therapeutic uses of Ocimum sanctum as the herb contains phytochemicals that have possess antioxidant, adaptogenic and immunomodulator properties [5]. Biochemical, microbiological and clinical investigation of plants have been making tremendous contributions to life sciences, health, and medicine. A double-blind trial conducted in 2011 suggested that an alcoholic extract of Tulsi modulates immunity, thus promoting immune system function [6]. The goal of the present study was to investigate and compare the nutritional quality of Tulsi leaves and flowers followed by focusing on the preparation of bioactive extract to evaluate cytotoxicity effect, antimicrobial, antifungal, insecticidal, and anti-diabetes properties of these extracts.
Determination of moisture
The moisture content was measured by the oven-drying method. About 5 gm of leaves and flowers were weighed in a porcelain crucible, which was (previously cleaned, heated to 100°C, cooled and weighed). The crucible with the sample was heated in an electrical oven for about 6 hours at 100°C. It was then cooled in desiccators and weighed again.
Percent of moisture content (gm per 100 gm of Ocimum sanctum leaves and flowers).
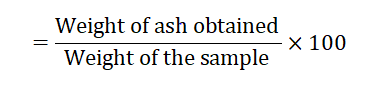
Determination of ash
Ash content was measured by the A.O.A.C method [7]. About 5 gm of Ocimum sanctum leaves and flowers were weighed in a porcelain crucible (which was previously cleaned and heated to about 100°C, cooled and weighed). The crucible was placed in a muffle furnace for about four hours at about 600. It was then cooled in a desiccator and weighed. To make sure completion of ash formation, the crucible was again heated in the muffle furnace for half an hour, cooled and weighed again. This was repeated until two consecutive weights were the same and the ash was almost white in color.
Percent of ash content (gm per 100 gm of Ocimum sanctum leaves and flowers)
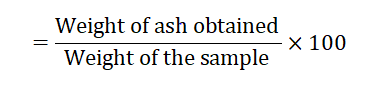
Extraction and estimation of chlorophyll in Ocimum sanctum leaves
Chlorophyll content of Tulsi leaves was measured by the following method described by Mahadevan and Shidhar [8].
Procedure: About 5 gm of leaves and flowers were cut into small pieces and homogenized well with excess acetone in a mortar with pestle and then filtered the extract through a Buchner funnel using Whatmann no.42 filter paper. Then sufficient quantity of 80% acetone was added and repeated the extraction. The content from the extraction was transferred to Buchner funnel and washed with 80% acetone until colorless. The filtrates were pooled and made up to the volume of 100 ml in a volumetric flask with 80% acetone. The absorbance of the extract was measured at 650 nm and 663 nm for determination of chlorophyll-a and chlorophyll-b respectively.
The chlorophyll contents were calculated on fresh weight basis employing the following formula as described as Mahadevan, using the specific absorption coefficients for chlorophyll-a chlorophyll-b at 663 nm and 645 nm in 80% acetone respectively.
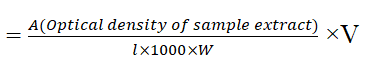
Chlorophyll-(mg/gm)
Where, A=Optical density in each case
l=length of light path in the cell (usually in cm)
V=Volume of the extract in ml, and
W=Fresh weight of the sample in gram.
Determination of water-soluble protein
Water soluble proteins content of Ocimum sanctum leaves and flowers were measured by the Lowry method [9].
Procedure: Extraction of juice from the leaves and flowers of Ocimum sanctum
About 5 gm of leaves and flowers of Ocimum sanctum was taken in a mortar with small amount of water. The supplied sample was crushed thoroughly with a pestle and then filtered through two layers of muslin cloth. The filtrate was then centrifuged for 10 minutes at 3000 r.p.m and the clear supernatant was collected.
Reagents:
a) 2% sodium carbonate solution in 0.1 N sodium hydroxide solution
b) 0.5% copper sulfate in 1% sodium-potassium tartarate
c) Folin-Ciocalt eau reagent (FCR)
Reagents (a) and (b) were mixed in the ratio of 50:1 and reagent (c) were diluted before use.
Procedure: A standard curve of protein was prepared by taking 0.0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.8 and 1.0 ml of standard protein solution in each test tube respectively and made the volume up to 1 ml with distilled water. The sample was transferred to a 50 ml volumetric flask and the volume was made up to the mark by distilled water. Water was carefully added avoiding formation of emulsion. 1 ml of the sample was taken in a test tube and a duplicate was made. To each of the tubes 5.0 ml of (a:b) mixture was added and after 10 minutes, 0.5 ml (FCR) solution was added. Absorbance of the solution was recorded after 30 minutes at 650 nm. A standard curve was constructed with the data obtained from the standard solution and the amount of protein in the sample was calculated using the standard curve (Figure 1).
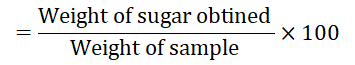
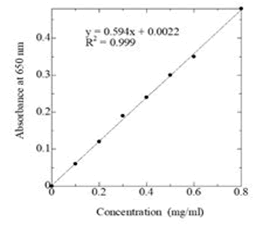
Figure 1: Standard curve for the determination of protein concentration be lower method.
Percent of protein content (mg per 100 gm of Ocimum sanctum leaves and flowers)
Determination of total soluble sugar
Total sugar content of Ocimum sanctum leaves and flowers were measured spectrophotometrically by the Anthrone method [10].
Extraction of sugar from Ocimum sanctum leaves and flowers were performed following the method described by Loomis and Shull.
About 5 gm of Ocimum sanctum leaves and stem were separately plunged into boiling ethyl alcohol and allowed to boil for 5-10 minutes (5 to 10 ml of alcohol was used per gm of flesh pulp) the extract was cooled and pasted thoroughly in a mortar with a pestle. Then extract was filtered through two layers of muslin cloth and re-extracted the pasted material for 3 minutes in hot 80% ethanol, using 2 to 3 ml of ethanol per gm of sample. This second extraction ensured complete removal of ethanol-soluble substances. The extract was cooled and passed through muslin cloth. Both the extracts were filtered through Whatmann No. 41 filter. The volume of the extract was evaporated to about one-fourth the volume over a steam bath and cooled. The concentrated extract was then transferred to a 100 ml volumetric flask and made up to the mark with distilled water. Then 1 ml of the diluted solution was taken into another 100 ml volumetric flask and made up to the mark with distilled water.
Procedure: Aliquot of 1 ml of the leaves and flower extracts were taken into test tubes and 4 ml of the Anthrone reagent was added to each test tube and mixed well. Glass marbles were placed on the top of each tube to prevent loss of water by evaporation. The test tubes were heated for 10 minutes in a boiling water bath and then cooled. A blank was prepared by taking 1 ml of water and 4 ml of Anthrone reagent in a test tube and treated similarly. The absorbance of the blue-green solution was measured at 680 mm by spectrophotometer.
A stander curve of glucose was prepared by taking 0.0, 0.1, 0.2, 0.4, 0.6, 0.8, and 1 ml of standard glucose solution in each test tubes containing 0.0, 10, 20, 40, 60, 80 and 100 mg of glucose respectively and made the volume up to 1 ml with distilled water. Then absorbance was measured at 380 nm using the blank. The amount of total sugar was calculated from the standard curve of glucose (Figure 2).
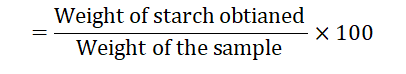
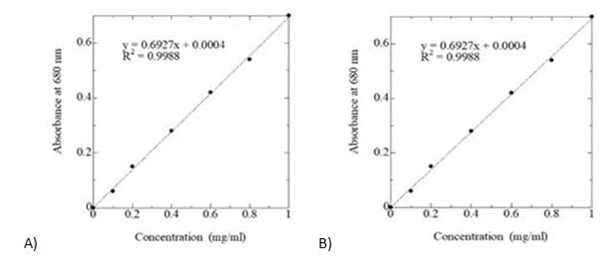
Figure 2: Standard curve of glucose for estimation of A) total soluble sugar B) starch.
Percent of total sugar (mg per 100 mg of Ocimum sanctum leaves and flower)
Determination of starch content of Ocimum sanctum leaves and flower
Then starch content of the Ocimum sanctum leaves and flower were measured by the Anthrone method.10
Reagents: Anthrone reagent (0.2% Anthrone in concentrated H2SO4), Standard glucose solution (10 mg/100 ml), and 1M HCl.
Procedure: About 5 gm of Ocimum sanctum leaves and flower were cut into small pieces and homogenized well with 20 ml of distilled water. The homogenate was then filtered through double layers of muslin cloth. Twice the volume of ethanol was added to the filtrate to precipitate the polysaccharide, mainly starch. Then it was kept overnight in cold; the precipitate was collected by centrifugation at 3,000 rpm for 15 minutes. The dried precipitate was then dried over a steam bath. Then 40 ml of 1M HC1 was added to the dried precipitate and heated to about 70°C. It was transferred to a volumetric flask and dilute to 100 ml with 1M HCl. Then 2 ml of dilute solution was taken into another 100 ml volumetric flask and made up to the mark with 1M HCl.
1 ml of the extract was placed into test tubes and 4 ml of Anthrone reagent was added to each tube and mixed well. Glass marbles were placed in a boiling water bath for 10 minutes, then removed and cooled. A blank was prepared by taking 1 ml of Anthrone reagent in a test tube and treated as before. The absorbance of the bluegreen solution was measured at 680 nm by spectrophotometer. The amount of starch present in the Ocimum sanctum leaves and flowers were calculated using the standard curve of glucose (Figure 2).
The percentage of starch (gm per 100 gm of Ocimum sanctum leaves and flowers)
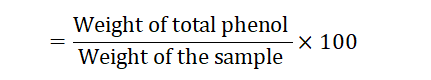
Determination of total phenol content of Ocimum sanctum leaves and flowers
Total phenol content Ocimum sanctum leaves and flowers were measured by Folin Cicalteau’s method [11].
Reagents: Folin–Ciocalteu reagent, 20% Sodium carbonate, and Catechol (0.1 mg/ml).
Extraction of phenol from Ocimum sanctum leaves and flowers were done following the method as described by Loomis and Shull.
Procedure: 5 gm of dried Ocimum sanctum leaves and flowers were cut into small pieces and immediately plunged into boiling ethyl alcohol and allowed to boil for 8-10 minutes (5 to 10 ml of alcohol was used per every gm of leaves). The extract was cooled and crushed through two-layer muslin cloth and re-extracted the tissue for 3 minutes in hot 80% ethanol using 2 to 3 ml of ethanolsoluble substances. The extract was cooled and passed through muslin cloth. Both the extract was filtered through Whatmann No. 41 filter paper.
Method: Aliquot of 1 ml of the extract was taken into test tubes. Then 1 ml of Folin-Ciocalteu reagent and 2 ml of Na2CO3 solution were added to each test tube and mixed well. The test tubes were placed in a boiling water bath for exactly two minutes, then removed and cooled. The blue solution was then transferred to a 25 ml volumetric flask and made up to the mark with distilled water. Then the solution was filtered. A blank was prepared by taking 1 ml of water and 1 ml of Folin-Ciocalteau reagent in a test tube and treated similarly. The absorbance of the blue solution was measured at 650 nm by spectrophotometer. The amount of total phenol was calculated from the standard curve of Catechol (Figure 3).
Total phenol content (mg per 100 gm of Ocimum sanctum leaves and Flowers)
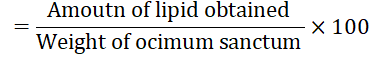
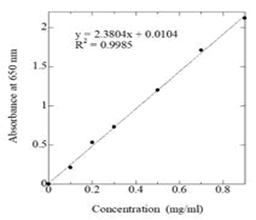
Figure 3: Standard curve of catechol for estimation of total phenol.
Determination of lipid content of Ocimum sanctum leaves and flowers
Lipid contents of Ocimum sanctum leaves and flowers were measured by the method of Bligh and dyer [12].
Procedure: 1 gram of Ocimum sanctum (leaves and flower) was first grounded in a mortar with about 10 ml distilled water. The grounded flesh was transferred into a separating funnel and added 30 ml of chloroform-ethanol and then kept overnight at room temperature in the dark. At the end of this period, 20 ml of chloroform and 20 ml of water were further added and mixed well. Generally, three layers were seen. A clear layer of chloroform containing all the lipids, a colored aqueous layer of ethanol with all water-soluble materials, and a thick pasty interphone were seen.
The chloroform layer was carefully collected in a per-weighed beaker (50 ml) and then placed on a steam bath for evaporation. After evaporation of the chloroform, the weight of the beaker was measured again. The difference in weight gives the amount of lipid.
Percent of lipid content (gram per 100 grams Ocimum sanctum leaves and flower)
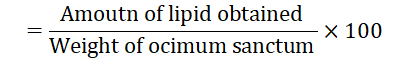
Determination of pectin content of Ocimum sanctum leaves and flowers
Pectin content of Ocimum sanctum was measured by the king’s gravimetric determination of pectin as calcium pectin [13].
Reagents: 95% Ethyl alcohol, 0.002M NaOH, 0.1M Acetic acid, and 10% CaCl2.
Procedure: 4-6 grams of Ocimum sanctum leaves were cut into small pieces and homogenized well with water and heated on a steam bath for 5-10 minutes in a 50 ml beaker. Then added 95% ethyl alcohol with little stirring and incubated the solution for about two hours at 50°C. Then the extract was filtered through two layers of muslin cloth and re-extracted the ground tissue for three times in hot 95% ethyl alcohol. Then added small excesses of 0.002M NaOH and allowed the solution to standard for one hour. Then, added 20 ml of 0.1M acetic acid and 40 ml of 10% CaCl2 solution and incubated for one hour, boiled and filtered through a Whatmann no. 41 filter paper. Then boiled with 40 ml of water and added 20 ml of 0.1M acetic acid. Finally, the viscous solution was dried and the amount obtained as calcium pectate.
The percentage of pectin as calcium pectate (gram per 100 grams of Ocimum sanctum leaves and flower)
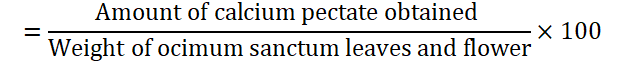
Crude fiber estimation of Ocimum sanctum leaves and flowers
Crude fiber was measured by the following method AOAC [7].
Reagents: H2SO4 (0.26N), NaOH (1.25%), Ethanol, and Ether.
Procedure: 5 grams of fat-free Ocimum sanctum leaves and flower were taken into 500 ml beaker and 200 ml of boiling 0.26N H2SO4, was added. Then the mixture was boiled for 30 minutes, keeping the volume constant by the addition of water at frequent intervals (a glass rod inserted in the beaker helped smooth). At the end of this period, the mixture was filtered through a muslin cloth and the residue was washed.
The extract was then transferred into the same beaker and 200 ml of boiling, 1.25% NaOH was added. After boiling for 30 minutes (keeping the volume constant as before), the mixture was filtered through muslin cloth. The extract was washed with hot water until free from alkali, followed by washing with some ethanol and ether. Then it was transferred to a crucible dried overnight at 80-100°C and weighed. The crucible was then heated in a muffle furnace at 600°C for 3 hours, cooled and weighed again. The difference in the weight represented the weight of crude fiber. The percentage of crude fiber (on dry basis) was calculated from the formula given below.
Crude fiber content (gram per 100 grams of Ocimum sanctum leaves and flower)
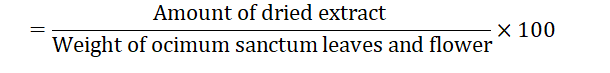
Determination of vitamin C content of Ocimum sanctum leaves and flowers
Vitamin C content of sample was measured by the Titramatric method [13].
Principle: Ascorbic acid (vitamin C) has acidic properties (donation of H+ ion). The acidity of vitamin C is not due to the carboxylic group-tied-up ion lactones form, but is due to the ionization of enol group. The method of the estimation of vitamin is based on the stoichiometric reduction of the dye 2, 6-dichlorophenol indophenol to colorless compound by ascorbic acid. The titration is conducted in the presence of metaphosphoric acid to inhibit the oxidation of ascorbic acid catalyzed by certain metallic iron (such as cupric and silver ion present in distilled water). In an aqueous system, this vitamin is easily oxidized and the stability increases with the increase in pH. Metaphosphoric acid stabilizes the solution by lowering the pH because of titration. Vitamin C is oxidized to dehydroascorbic acid.
Reagents:
a) Dye Solution: 200 mg of 2, 6-dichlorophenol and 210 mg of sodium bicarbonate were dissolved in distilled water and made up to 100 ml. The solution was filtered.
b) 3% Metaphosphoric acid reagent: 3 gm of metaphosphoric acid was dissolved in 80 ml of acetic and made up to 100 ml with distilled water.
c) Standard Vitamin C Solution (0.1 gm/ml): 10 mg of pure vitamin C was dissolved in 3% metaphosphoric acid and made up to 100 ml with 3% Metaphosphoric acid.
Procedure: 10 ml of standard vitamin C solution was taken in a conical flask and titrate it with the dye solution. About five grams of sample were cut into small pieces and homogenized well with 3% metaphosphoric acid (approximately 30 ml) and filtered it through double layer of muslin cloth. The filtrate was centrifuged at 3000 r.p.m. for 10 minutes and clear supernatant was titrated against 2, 6-dichlorophenol-endophenol solutions. The amount of vitamin C present in the extract was determined by comparing with the titration result of standard vitamin C solution.
Vitamin C content of the sample was calculated by the following equation 10 ml Standard Vitamin C Solution=1 mg of vitamin C per 100 gm sample
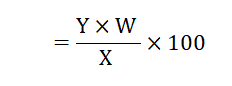
X=Volume of sample solution required to titrate the dye solution
Y=Volume of standard vitamin C Solution required to titrate the dye solution
W=Weight of sample taken
Determination of Vitamin B1 content of Ocimum sanctum leaves and flower
Vitamin B1 content of Ocimum sanctum leaves and flower were measured by the method of Anon [14].
Reagents: 2% Potassium ferrieyanide, Oxidizing reagents: 10 ml of 2% potassium ferrieyanide was mixed with 3.5N NaOH solution (90 ml), Quinine sulphate, 0.2N HC1, and Alcohol.
Preparation of standard thiamine hydrochloride solution:
5 mg of thiamine hydrochloride was transferred into 100 ml volumetric flask and dissolved in 30 ml of dilute alcohol solution and made up to the mark. The PH was adjusted to 4.0 with dilute HCl and stored in a light-resistant container.
Procedure: 5-6 grams of fresh Ocimum sanctum’s leaves and flower were cut into small pieces and homogenized with 0.2N HCl. The mixture was heated on a steam bath and then cooled. 5 ml of standard thiamine-HCl solution was mixed rapidly with 3 ml oxidizing reagent and 20 ml of isobutyl alcohol was added within 30 seconds, and then mixed the mixture vigorously for 90 seconds by shaking the tubes manually. A blank was prepared only by substituting the oxidizing reagent with an equal volume of 3.5N sodium hydroxide and proceed in the same manner. 21M of dehydrate alcohol was added, swirl for few seconds, allowed the phase to be separated and decanted or drawn off and transferred into cuvettes, then measured the fluorescence. Ocimum sanctum leaves and flower extract (5 ml) was pipette in different test and in the same manner as described above.
The amount (in milligram) of thiamine hydrochloride in each 5 ml of the Ocimum sanctum leaves and flower extract were calculated from the formula (A-b)/(S-d), in which A and S were the average fluorometer reading of the portions of Ocimum sanctum leaves and flower extracts and standard preparation with oxidizing reagent, respectively, and b and d were the reading for the blanks of Ocimum sanctum leaves and flower were extracts and standard preparation, respectively.
The percentage of vitamin B1 (mg per 100 mg of fresh Ocimum sanctum leaves and flower)

Determination of vitamin B2 content of Ocimum sanctum leaves and flower
Vitamin B2 content of Ocimum sanctum leaves and flower were measured by the method of Anon [14].
Reagents: 0.02N Acetic acid, 0.1N H2SO4, 0.1N NaOH, 0.1N HCl, 4% Potassium permanganate, and Hydrogen peroxide.
Procedure: Preparation of standard riboflavin: 50 mg of riboflavin was mixed with 300 ml of 0.02N acetic acid and the mixture was heated on a steam bath with frequent agitation until the riboflavin was dissolved. Then cooled and made up to 500 ml with 0.02N acetic acid. This solution was diluted appropriately with 0.02N acetic acid. This solution was diluted appropriately with 0.02N acetic acid to made final riboflavin concentration of 10 μg/ml.
Extraction of riboflavin from Ocimum sanctum leaves and flower: Fresh Ocimum sanctum leaves and flower (5-6 grams) were cut into small pieces and homogenized well with 0.1N H2SO4 (about 50 ml). The mixture was heated in an autoclave at 121-123°C for 30 minutes, then cooled it and filtered through double-layer of muslin cloth. The filtrate was made up to 100 ml with distilled water and 25 ml of this solution was taken into a beaker and then added 25 ml water. The mixture was agitated vigorously and adjusted the pH 6.0 to 6.5 with 0.1N NaOH. Immediately, 0.1N HC1 was added until no preparation occurs. The extract was again filtered and pH of the extract was adjusted to 6.6 to 6.8 with 0.1N NaOH.
10 ml of Ocimum sanctum leaves and flower were taken into the test tube, add 1.0 ml of water; 1.0 ml of glacial acetic acid was added to it. The mixture was then mixed with 0.5 ml of potassium permanganate solution and allowed to stand for two minutes, and then 0.5 ml of hydrogen peroxide solution was added, where upon the permanganate color was destroyed within 10 seconds. The tube was shaken vigorously until excess oxygen expelled, then 1 ml of standard solution was pipette in a test tube and treated in the same manner as that described for the sample extract. In a suitable fluorometer, the fluorescence of the tubes was measured. Then 20 mg of sodium hydrosulphite were added to each test tube and mixed well. Finally measured the fluorescence within 5 seconds.
The quantity in milligram in each ml of the Ocimum sanctum leaves and flower were calculated by the following formula, 0.0001 (Iu-IB) (IS-IU)
Where, Iu=Average reading for Ocimum sanctum leaves and flower
Is=Average reading for standard preparation
IB=Average reading for mixed with sodium hydrosulphite.
Mg percentage of vitamin-B2 content in Ocimum sanctum leaves and flower (mg per 100 gm Ocimum sanctum leaves and flower)

Determination of mineral content of Ocimum sanctum leaves and flowers
Organic matter was digested and Ca, Fe and Zn were released by digestion with nitric acid. Ca, Fe, and Zn were measured by atomic absorption spectrophotometer [15].
Reagents: Iron accelerator, Copper accelerator, Concentrated H2SO4, Catalyst mixture, 33% NaOH, 0.05M NaOH, 0.05 M HCl, Methyl red–methyl blue indicator solution, 68% Nitric acid, 1:20 diluted HNO3, 1:100 diluted HNO3, 5M HNO3, CaCl2-solution, Acetate buffer solution, Azomethine-H reagent, Perchloric acid, HCl, 0.5M Barium chloride, and Silver nitrate.
Used two stock solutions and slandered solution of each mineral at different concentration.
Digestion: 0.5 g dried plant material was weighed into each of 38 nitrogen digestion tubes. The two remaining tubes were blanks. 5 ml 68% nitric acid was added to each of all 40 tubes. The content in each tube was mixed and left overnight. The tubes in the digester were placed and covered the tubes with the exhaust manifold. The temperature was set to 125°C. The digester was turn of and continued the digestion for 4 hours after boiling has started. Precaution was taken so that no tubes become dry. After cooling the digestion mixture was transferred with distilled water to a 100 ml volumetric flask. The flask was made up to volume with water and mixed and filtered on a dry filter into a dry bottle, which was closed with a screw cap. The filter was kept in the closed bottle. Ca, Fe and Zn content in the filtrates were measured.
Estimation of Ca, and Zn: 20 ml diluted filtrate was transferred into a 50 ml volumetric flask and the flask was made up to volume with distilled water and mixed. The content of Ca and Zn were measured by atomic absorption spectrometer (AAS). If the reading of sample is higher than the reading of the highest standard solution, then it is need to larger dilution, e.g., 10 ml filtrate into a 50 ml volumetric flask. In this case 1:10 diluted HNO3 and filtrate equal to 20 ml.
Estimation of P: 5 ml dilute filter was transferred to a 50 ml volumetric flask. Water (approximately 30 ml), added to it and mixed well followed by addition of 10 ml ammonium molybdateascorbic acid solution. The flask was made up to volumetric with water and mixed. After 15 minutes the absorbance on a spectrophotometer at 890 nm was measured. If the absorbance was higher than that of the highest standard solution, the procedure was repeated using a smaller amount of filtrate. In the case 1:100 HNO3 and filtrate equal to 5 ml.
Estimation of Fe: The content of Fe was measured by atomic absorption spectrometer (AAS) directly in the undiluted filter.
Ca, Zn and P mg per kg plat material
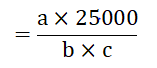
Where,
a=mg/l Ca, Zn or P measured of atomic absorption spectrometer, flame photometer of spectrophotometer,
b=ml dilute filtrate transferred into the 50 ml volumetric flask for determination of Ca, Zn and P.
c=Amount of plant material (g) weighted into the digestion tube.
If an additional dilution is made before the transfer to the 50 ml volumetric flask, the result is multiplied by the dilution factor.
Fe mg per kg plant material
Where,
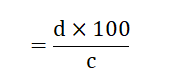
D=mg/l Fe measured on atomic absorption spectrophotometer
C=g plant material weighted into the digestion tube
Sterilization procedure: Antimicrobial screening was carried out in a laminar airflow unit and all types of precautions were highly maintained to avoid any contamination during the test. UV light was switched on one hour earlier of the start of the experiment to avoid contamination. Petri dishes and other glassware were sterilized by an autoclave machine at a temperature of 121°C and pressure of 15 lb/sq inch for 30 minutes. Blank discs were also sterilized and kept in laminar hood under UV light for 30 minutes.
Preparation of the culture media: For demonstrating the antibacterial activity and subculture of the test organisms, nutrient agar media (Difco) was used. For preparation of 100 ml nutrient agar media, 0.5 gm peptone, 1gm yeast extract, 0.5 gm sodium chloride and 2 gm agar were dissolved in distill water.
Preparation of fresh culture: The nutrient agar medium was prepared and dispersed in a number of clean test tubes to prepare slants (5 ml in each test tube). The test tubes were plugged with cotton and sterilized for 30 minutes. After sterilization, the test tubes were kept in an inclined position (45°C) for solidification. These were then incubated at 37.5°C to ensure sterilization. The test organisms were transferred to the agar slants from the supplied pure cultures with the help of an inoculating loop in an aseptic condition. The loop was red heated carefully after each transfer of microorganism to avoid contamination. The inoculated slants were then incubated at 37.5°C for 24 hours to assure the growth of test organisms. These fresh cultures were used for the sensitivity test.
Preparation of the test plates: The test plates were prepared according to the following procedure.
a) 15 ml previously prepared nutrient agar medium was poured in each of the clean test tubes and plugged with cotton.
b) The test tubes and a number of Petri dishes were sterilized in an autoclave for 30 minutes and were transferred into laminar air flow unit and then allowed to cool to about 45°C to 50°C.
c)_The test organism was transferred from the fresh subculture to the test tube containing 15 ml autoclaved medium with the help of an inoculating loop in an aseptic condition. Then the test tube was gently shaken to get a uniform suspension of the organism.
d) The bacterial suspensions were immediately transferred to the sterile petridishes in an aseptic area. The petridishes were rotated several times, first clockwise and then anticlockwise to assure homogenous distribution of the test organisms.
e) The media were poured into Petridishes in such a way as to give a uniform depth of approximately 4 mm.
f) Finally, the medium was cooled at room temperature under laminar airflow and was stored in a refrigerator (4°C).
Sample discs: Sterilized (BBL, U.S.A) filter paper discs (5 mm in diameter) were prepared with the help of punch machine and were taken in a blank petridish. Sample solution of desired concentration (10 μl/disc) was applied on the discs with the help of a micropipette in an aseptic condition.
Standard discs: Standard discs were used to compare the antibacterial activity of test materials. Kanamycin (30 μg/discs) was used as standard disc.
Solvent control discs: Solvent control discs were prepared using same filter paper (5 mm diameter) and same volume of residual solvent without sample following the same process and condition. These were used as negative control to ensure that the residual solvent and the filter paper themselves was not active.
Preparation of test sample: 15 mg and 20 mg of crude ethanol extract were dissolved in 1 ml ethanol in separate glass vial. Thus, the concentrations were 30 μg/μl, 40 μg/μl respectively for each extract.
Placement of the discs and incubation: The following procedure was adopted for the placement of the discs
Firstly, the dried crude extract discs and standard discs were placed gently on the solidified agar plates seeded with the test organisms with the help of a pair of sterile forceps to ensure contact with the medium.
Secondly, the plates were kept in a refrigerator at 4°C for 24 hours in order to provide sufficient time to diffuse the antibiotics into the medium.
Finally, the plates were incubated at 37.5°C for 24 hours in an incubator.
Measurement of the zones of inhibition: After incubation, the antibacterial activities of the test samples were determined by measuring the diameter of inhibitory zones in term of mm with a transparent scale.
Fungal culture
The ethanol extracts were used for the antifungal activity study. The test sample of ethanol extracts of Ocimum sanctum (10 mg, 15 mg, and 20 mg) was dissolved in 1 ml of respective solvent separately to get concentrations of 10 mg/ml, 15 mg/ml and 20 mg/ml respectively. The Nystatin 10 mg was also dissolved in 1 ml of ethanol that serving as control with concentration of 10 mg/ml.
Preparation of culture media: Potato Dextrose Agar (PDA) media was used to perform the antifungal activity test and for subculture of the test organism. The composition of the media was as follows.
Peeled and sliced potato=200 gm
Dextrose=40 gm
Agar=20 gm
Distill water=1000 ml
Preparation of the test plates: The test plates were prepared according to the following procedure.
a) About 10 ml in quantity of distilled water was poured in several clean test tubes and plugged with cotton.
b) The test tubes, a number of Petri dishes, glass rods, a piece of cotton and the medium were sterilized by autoclave and then transferred to the laminar air flow cabinet.
c) About 6 ml of the medium was poured carefully in the medium sized Petri dishes in each. The Petri dishes were rotated several times, first clockwise and then anticlockwise to assure homogenous thickness of the medium and allowed to cool and solidify at about 30°C.
d) The test tubes containing distilled water were inoculated with fresh culture of the test fungi and were shaken gently to form a uniform suspension of the organism because of their high prevalence sporulation process.
e) A piece of cotton was immerged in the test tubes with the help of individual glass rod and then gentle rubbed the medium and the cotton was discarded.
f) Finally, the plates were stored in a refrigerator (4°C) for overnight.
Preparation of discs: Two types of antifungal discs were prepared for antifungal screening.
a) Sample discs (Ethanol extracts): Sterilized metrical (BBL, Cocksrvile, USA) filter paper discs (4 mm diameter) were taken in a blank Petri dish. Sample solution of desired amount (10 ml/ disc) was applied on the discs with the help of micropipette in and aseptic condition. The discs were left for a few minutes in an aseptic condition for complete removal of solvent.
b) Standard discs (Nystatin): It was also prepared by the same process of sample discs preparation. Thus, the concentration of standard Nystatin was 100 mg/disc.
Placement of the discs and incubation: For the placement of the discs, the following procedure was adopted:
a) The dried crude extract discs and standard disc were placed gently on the solidified agar plates seeded with the test organisms to ensure contact with the medium.
b) The plates were then kept in a refrigerator at 4°C for 24 hours in order to provide sufficient time to diffuse the antibiotics into the medium.
c) Finally, the plates were incubated at 37.5°C for 24 hours in an incubator.
Measurement of the zones of inhibition: After incubation, the antibacterial activities of the test samples were determined by measuring the diameter of inhibitory zones in term of mm with a transparent scale.
Animal study: Healthy Wister Albino rats (Rattus norvegicus) of either sex, weighting between 150-250 gm were used for the study. These rats were collected from International Centre for Diarrheal Diseases Research, Bangladesh (ICDDR’B). Animals were kept in standard polypropylene cage and maintained under standard laboratory conditions of temperature (25 ± 10C), relative humidity (50 ± 15%), 12-hour light and dark cycle and maintained Institutional Animal Ethical Committee (IAEC). Streptozotocin was purchased from Science laboratory store and injected intraperitoneally (60 mg/kg body weight) to induce diabetes in rats. The streptozotocin solution was freshly prepared dissolving in citrate buffer (0.1M, PH 4.5)
Moisture and ash content of Ocimum sanctum leaves and flowers
The moisture content of Ocimum sanctum leaves and flowers were found about 84.78% and 71.56% respectively as shown in Table 1. The result reveals that the moisture content of Ocimum sanctum leaves greater than flowers. Moisture is necessary for most of the physiological reaction in plant tissues. Moisture contributes essential properties of life as do the other elements such as carbohydrate and protein. Most of the inorganic constituents or mineral are present in ash. The ash content of Ocimum sanctum leaves and flowers are given in the Table 1.
| Constituents | Leaves | Flowers |
|---|---|---|
| Moisture (%) | 84.78% | 71.56% |
| Ash (%) | 12.72% | 25.56% |
Table 1: Moisture and ash content in Ocimum sanctum leaves and flowers.
The ash content was observed 12.72% and 25.56% leaves and flowers respectively. This result suggests that the ash content is greater in flowers than the leaves.
Water soluble protein content of Ocimum sanctum leaves and flowers
The amount of water-soluble protein present in the Ocimum sanctum leaves and flowers are shown in the Table 2. The Ocimum sanctum leaves and flowers contained 50% and 24.6% protein respectively.
| Constituents | Leaves | Flowers |
|---|---|---|
| Water-soluble protein (mg %) | 40.60% | 17.77% |
| Lipid (mg %) | 0.50% | 0.02% |
| Crude fibers (mg %) | 14.80% | 5.38% |
| Total phenol (mg %) | 0.37% | 0.25% |
Table 2: Water-soluble protein, Lipid, Crude fibers and phenol contents in the Ocimum sanctum leaves and flowers.
It indicates the highest amount of water-soluble protein present in leaves and flowers of Ocimum sanctum. Protein plays an important role in all the biological processes.
Lipid content of Ocimum sanctum leaves and flowers
Fats are concentrated form of energy and they play an important role as carrier of certain fat-soluble vitamins. Lipid serves as important source of energy, maintains cell structure, reproduction and embryogenesis. Lipid contents of Ocimum Sanctum leaves and flowers are shown in Table 2. The result clearly indicates that Ocimum Sanctum leaves and flowers contained little amount of lipid.
Crude fibers content of Ocimum sanctum leaves and flowers
Cellulose, lignin are the components of crude fibers. In leaves cellulose are present in cell wall. It is commonly used as measure of the nutritional value of poultry and livestock feeds. Crude fiber plays an important role for preventing cancer. The crude fibers content of Ocimum sanctum leaves and flowers are shown in the Table 2. The amount of crude fibers present in Ocimum sanctum leaves and flowers are 14.8% and 5.38% respectively.
Total phenols content of Ocimum sanctum leaves and flowers
Phenolic compounds are distributed in the plant kingdom. They are particularly prominent in fruits and vegetables where they are important in determining color and flavor. Total phenol content of Ocimum sanctum leaves and flowers shown in Table 2. The Ocimum sanctum leaves and flowers contain 12.6% and 6.6% total phenol respectively.
Total sugar content of Ocimum sanctum leaves and flowers
Total sugar content of Ocimum sanctum leaves and flowers are given in Table 3. The amount of total sugar present in Ocimum sanctum leaves and flowers were 60% and 77% respectively.
| Constituents | Leaves | Flowers |
|---|---|---|
| Total sugar content (mg %) | 60% | 77% |
| Starch (mg %) | 13.90% | 16.10% |
| Pectin (mg %) | 7.82% | 5.32% |
Table 3: Total sugar, starch and pectin content in Ocimum sanctum leaves and flowers.
Starch content of Ocimum sanctum leaves and flowers
The amount of starch content of Ocimum sanctum leaves and flowers is 13.90% and 16.10% respectively. Starch content of Ocimum sanctum leaves and flowers are shown in Table 3.
Pectin content of Ocimum sanctum leaves and flowers
Pectin is polymer consisting largely of galacturonic acid, galactose and arabinose and is classified as gum. Plant tissue contains protopectin (which are chemical hemicelluloses) cementing the cell wall together. Pectin content of Ocimum sanctum leaves and flowers is 7.82 mg/100 grams and 5.33 mg/100 grams respectively. The calcium pectate present in Ocimum sanctum leaves and flowers are present in Table 3.
Vitamin C content of Ocimum sanctum leaves and flowers
The result shows that the vitamin C content of leaves was lower 14% compared to the flowers about 42%. The amount of vitamin C present in Ocimum sanctum leaves and flowers shown in the Table 4.
| Vitamins | Leaves | Flowers |
|---|---|---|
| Vitamin B1 | 0.48% | 0.27% |
| Vitamin B2 | 0.24% | 0.33% |
| Vitamin C | 14% | 42% |
Table 4: Vitamin content of Ocimum sanctum leaves and flowers.
Vitamin B1 content of Ocimum sanctum leaves and flowers
The result shows that the vitamin B1 content of leaves was 0.48 mg/100 g and flower 0.27 mg/100 grams. The amount of vitamin B1 present in Ocimum sanctum leaves and flowers shown in the Table 4.
Vitamin B2 content of Ocimum sanctum leaves and flowers
Vitamin B2 also called riboflavin. The result shows that the vitamin B2 content of leaves and flowers was 0.24% and 0.33% respectively. The amount of vitamin B2 present in Ocimum sanctum leaves and flowers shown in Table 4.
Mineral content of Ocimum sanctum leaves and flowers
Minerals are inorganic elements exist as organic and inorganic combination. Calcium content of Ocimum sanctum leaves and flowers were 25% and 17% respectively. Phosphorus contents of Ocimum sanctum leaves and flowers were 287 mg/100 grams and 167 mg/100 grams respectively. The iron content of Ocimum sanctum leaves and flowers were 15.1 mg/100 grams and 0.9 mg/100 grams respectively. Zinc content of Ocimum sanctum leaves and flowers were 20 mg/100 grams and 18 mg/100 grams respectively. The amount of calcium, zinc, iron, phosphorus present in Ocimum sanctum leaves and flowers shown in Table 5.
| Minerals | Leaves | Flowers |
|---|---|---|
| Ca | 25 mg/100 grams | 17 mg/100 grams |
| Zn | 20 mg/100 grams | 18 mg/100 grams |
| Iron | 15.1 mg/100 grams | 0.9 mg/100 grams |
| Phosphorus | 287 mg/100 grams | 167 mg/100 grams |
Table 5: mineral contents of Ocimum sanctum leaves and flowers.
Determination of antibacterial activity
In the present investigation, the efficacy of ethanol extract isolated from leaves Ocimum sanctum to inhibit the growth of three gram (+) positive bacteria (Staphylococcus aureus, Bacillus subtilis and Sarcina lutea) and three gram (-) negative bacteria (Pseudomonas aeruginosa, Salmonella typhi and Klebsiella pneumoniae) were studied at the doses of 300 g/disc and 400 μg/disc. The results obtained are shown in Tables 6 and 7 and Figures 4 and 5. EAE did not show any activity against Staphylococcus aureus, Bacillus. Standard Kanamycin (at 30 μg/disc dose) produced inhibition zone against Staphylococcus aureus, Bacillus subtilis, Sarcina lutea, Pseudomonas aeruginosa, Salmonella typhi and Klebsiella pneumoniae.
| Test bacteria | Ethanol extract of Ocimum sanctum leaves (µg/disc) | Kanamycin (µg/disc) | |
|---|---|---|---|
| 300 | 400 | 30 | |
| Zone of inhibition (diameter in mm.) | |||
| Staphylococcus aureus | 9 | 13 | 21 |
| Bacillus subtilis | 10 | 11 | 24 |
| Sarcina lutea | 9 | 12 | 33 |
| Pseudomonas aeruginosa | 8 | 11 | 27 |
| Salmonella typhi | - | - | 36 |
| Klebsiella pneumoniae | - | - | 27 |
| Note: ‘-`=No sensitivity | |||
Table 6: In vitro antibacterial activity of ethanol extract of Ocimum sanctum leaves and Kanamycin.
| Test bacteria | Ethanol extract of Ocimum sanctum flowers (µg/disc) | Kanamycin (µg/disc) | |
|---|---|---|---|
| 300 | 400 | 30 | |
| Zone of inhibition (diameter in mm.) | |||
| Staphylococcus aureus | 10 | 11 | 21 |
| Bacillus subtilis | 9 | 11 | 25 |
| Sarcina lutea | 8 | 12 | 33 |
| Pseudomonas aeruginosa | 11 | 13 | 35 |
| Salmonella typhi | - | - | 36 |
| Klebsiella pneumoniae | - | - | 29 |
| Note: ‘-`=No sensitivity | |||
Table 7: In vitro antibacterial activity of Ethanol extract of Ocimum sanctum flowers and Kanamycin.
Figure 4: Antibacterial activity of ethanol extract of Ocimum sanctum leaves against A) Sarcina lutea B) Staphylococcus aureus C) Pseudomonas aeruginosa D) Bacillus subtilis. SD=Standard (Kanamycin-30 µg/disc); S =Sample (400 µg/disc); S2=Sample (300 µg/disc); S=Solvent Control.
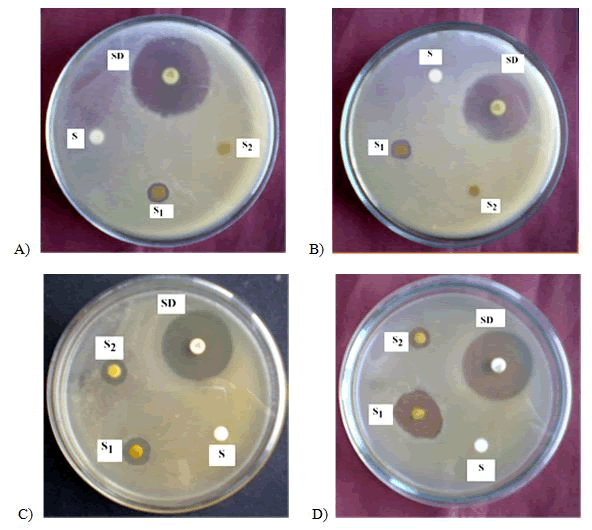
Figure 5: Antibacterial activity of ethanol extract of Ocimum sanctum flowers against A) Bacillus subtilis B) Sarcina leutea C) Staphylococcus aureus D) Pseudomonas aeruginosa. SD=Standard (Kanamycin-30 µg/disc); S =Sample (400 µg/disc); S2=Sample (300 µg/disc); S=Solvent Control.
The produced zone of inhibition for ethanol extract of Ocimum sanctum leaves against Staphylococcus aureus, Bacillus subtillis, sarcina lutea and pseudomonas aeruginosa were 9, 10, 9, 8 mm at 300 μg/ disc respectively whereas at 400 μg/disc dose, they produced zone of inhibition against the same bacteria were 13, 11, 12, 11 mm respectively.
On the other hand, ethanol extract of Ocimum sanctum flowers showed the activity against Staphylococcus aureus, Bacillus subtillis, Sarcina lutea, and Pseudomonas aeruginosa were 10, 9, 8, and 11 mm at 300 μg/disc respectively whereas at 400 μg/disc dose, the produced zone of inhibition against the same bacteria were 11, 11, 12, and 13 mm respectively. All these extracts had no activity against Salmonella typhi, Klebsiella pneumonia, Streptococcus haemolytia.
Plant derived phytochemicals serve as a prototype which develop the more effective drugs in controlling the growth of microorganisms. These compounds have significant therapeutic applications against some pathogenic bacteria and fungi. There are numerous studies that have been conducted with the extracts of various plants, screening antimicrobial activity as well as for the discovery of new compounds. From the above experiments, we found that the extracts of Ocimum sanctum leaves and flowers might have some compounds, which are responsible for antimicrobial activity.
Determination of antifungal activity
The antifungal activities of each extract against five pathogenic fungi were investigated by using the doses of 400 μg/disc and 500 μg/disc. The standard antibiotic disc of Nystatin (100 μg/disc) was used for comparison. The results of antifungal activity (zone of inhibition) of test materials against respective fungi were given in the Tables 8 and 9.
From the Tables 8 and 9 and Figures 6 and 7 it was found that ethanol extracts of leaves and flowers of Ocimum sanctum showed high antifungal activity against Aspergillus niger, Candida albicans and Saccharomyces cerevisiae at the dose of 500 μg/disc the produced zone of inhibition were 11 mm, 10 mm and 12 mm, while at 400 μg/disc the produced zone of inhibition were 8 mm, 9 mm and 10 mm respectively.
| Test fungi | Ethanol extract of leaves | Nystatin (100 mg/disc) | |
|---|---|---|---|
| (400 mg/disc) | (500 mg/disc) | ||
| Â Penicilium sp | - | - | 25 |
| Aspergillus niger | 8 | 11 | 24 |
| Saccharomyces cerevisiae | 10 | 12 | 26 |
| Candida albicans | 9 | 10 | 24 |
| Rizopus sp | - | - | 23 |
Table 8: In vitro antifungal activities of ethanol extract of leaves and Nystatin.
| Test fungi | Ethanol extract of flower | Nystatin (100 mg/disc) | |
|---|---|---|---|
| (400 mg/disc) | (500 mg/disc) | ||
| Â Penicilium sp | - | - | 26 |
| Aspergillus niger | 10 | 11 | 26 |
| Candida albicans | 9 | 12 | 25 |
| Saccharomyces cerevisiae | 8 | 10 | 26 |
| Rizopus sp | - | - | 24 |
Table 9: In vitro antifungal activities of ethanol extracts of flower and Nystatin.
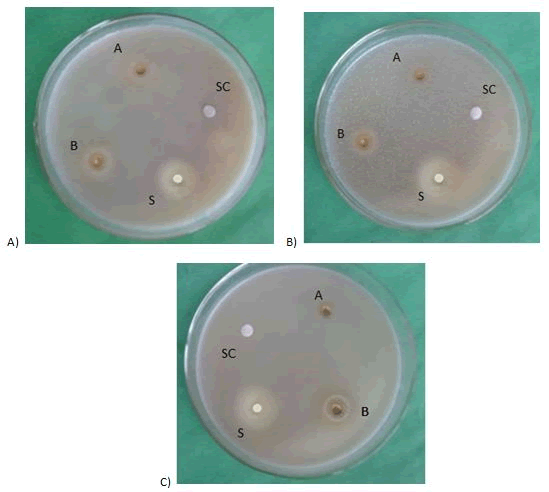
Figure 6: Effect of ethanol extract of leaves on A) Aspergillus niger B) Candida albicans C) Saccharomyces cerevisiae. SC=Solvent control; A=400 μg/disc; B=500 μg/disc; S=Nystatin (100 μg/disc).
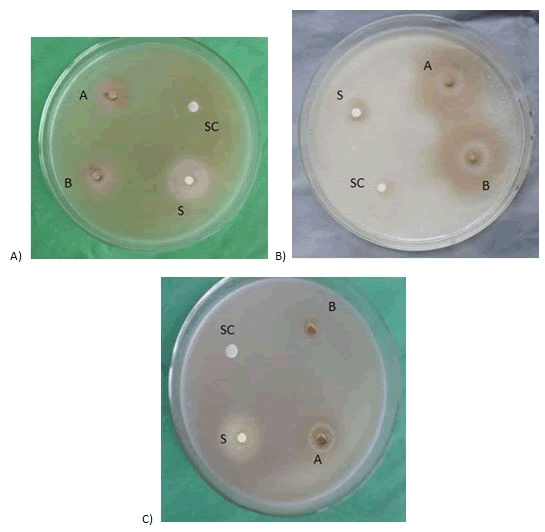
Figure 7: Effect of ethanol extract of flowers on A) Aspergillus niger, B) Candida albicans, C) Saccharomyces cerevisiae Miosporodis. SC=Solvent control; A=400 μg/disc; B=500 μg/disc; S=Nystatin (100 μg/disc).
The ethanol extract of flowers produced zone of inhibition were 11 mm, 12 mm and 10 mm at the dose of 500 μg/disc, while at the 400 μg/disc, the produced zone of inhibition were 10 mm, 9 mm and 8 mm respectively.
It was found that, ethanol extracts of both leaves and flowers showed no zone of inhibition against Penicilium sp and Rizopus sp.
In vitro antifungal screening is a useful technique for the detection of new lead compounds as potential antibiotics. From the investigations, we can conclude that ethanol extracts of Ocimum sanctum leaves and flowers were tested against five fungi by using disc diffusion technique. These extracts possess significant antifungal activity on microorganism due to the presence of various bioactive materials and it could be the source of new antibiotic compound treating different infections caused by pathogenic fungi.
Determination of antidiabetic activity
Effect on body weight: The body weight of diabetic rats was significantly decreased compared to control rats and was about 195.55 ± 3.96 and 210.77 ± 3.99 respectively. Ocimum sanctum leaves extract treated diabetic rats showed significant weights gains on 21st day relative to initial day and were about 212.54 ± 3.99 and 224.8 ± 3.66 respectively in 200 mg/kg and 400 mg/kg doses. On the other hand, flowers extract treated diabetic rats there were significant weights gains on 21st day relative to initial day and were about 220.54 ± 3.99 and 227.99 ± 3.39 respectively in 200 mg/kg and 400 mg/kg doses. The effect of leaves and flowers extracts as shown in Tables 10 and 11.
| Groups | Treatment | Body weight (gm) | |||
|---|---|---|---|---|---|
| Initial | 7th Day | 14th Day | 21th Day | ||
| I | Normal control | 190 ± 2.96 | 195.4 ± 3.65 | 202.85 ± 3.54 | 210.77 ± 3.99 |
| II | Diabetic control | 212.53 ± 2.48 | 204.63 ± 4.87 | 203.6 ± 2.83 | 195.55 ± 3.96 |
| III | LEOS (200 mg/kg) | 215.25 ± 3.11 | 210.6 ± 3.58 | 211.33 ± 3.22 | 212.54 ± 3.99 |
| IV | LEOS (400 mg/kg) | 216.9 ± 3.55 | 220.5 ± 2.92 | 223.7 ± 3.55 | 224.8 ± 3.66 |
| V | FEOS (200 mg/kg) | 217.25 ± 3.12 | 218.55 ± 3.96 | 219.02 ± 3.25 | 220.54 ± 3.99 |
| VI | FEOS (400 mg/kg) | 221.2 ± 3.44 | 223.02 ± 2.78 | 225.66 ± 3.22 | 227.99 ± 3.39 |
| VII | Glibenclamide (10 mg/kg) | 213.30 ± 3.95 | 215.23 ± 3.24 | 217.9 ± 3.52 | 219.86 ± 3.33 |
| Note: LEOS- Leave extract of Ocimum sanctum; FEOS- Flower extract of Ocimum sanctum. All values represent means ± S.D. | |||||
Table 10: Effect of Ocimum sanctum leaves and flowers extract on body weights in diabetic rats.
| Groups | Treatment | Body weight (gm) | |||
|---|---|---|---|---|---|
| Initial | 7th Day | 14th Day | 21th Day | ||
| I | Normal control | 90.12 ± 1.28 | 92.16 ± 1.6 | 95.21 ± 2.11 | 96.85 ± 3.33 |
| II | Diabetic control | 295.11 ± 1.22 | 298.55 ± .22 | 310.77 ± 0.9 | 400.11 ± 0.8 |
| III | LEOS (200 mg/kg) | 321.12 ± 2.2 | 233 ± 1.22 | 167 ± 1.0 | 140.9 ± 0.7 |
| IV | LEOS (400 mg/kg) | 324.22 ± 1.4 | 256.6 ± .7 | 166.4 ± 0.6 | 119.3 ± 1.3 |
| V | FEOS (200 mg/kg) | 330.3 ± .9 | 260.2 ± 1.3 | 166.62 ± 1.1 | 133.66 ± 0.5 |
| VI | FEOS (400 mg/kg) | 300.22 ± 1.0 | 224.77 ± 0.9 | 143.32 ± 2.0 | 109.33 ± 0.6 |
| VII | Glibenclamide (10 mg/kg) | 323.3 ± 1.2 | 237.45 ± 2.1 | 137.33 ± 1.3 | 98.77 ± 0.66 |
| Note: LEOS- Leave extract of Ocimum sanctum; FEOS- Flower extract of Ocimum sanctum. All values represent means ± S.D. | |||||
Table 11: Effect of Ocimum sanctum leaves and flowers extract on body glucose level in diabetic rats.
Effect on blood glucose level: There was a significant increase in blood glucose level in streptozotocin-induced diabetic rats when compared with normal control. Daily oral administration of the test extracts for 21 days significantly decreased blood glucose levels in streptozotocin-induced diabetic rats compared to vehicle-controls. The blood glucose levels were 140.9 ± 0.7 and 119.3 ± 1.3 mg/ dl at the dose of 200 and 400 mg/kg of alcoholic extract Ocimum sanctum leaves respectively. Besides, blood glucose level was 133.66 ± 0.5 and 109.33 ± 0.6 mg/dl at the dose of 200 and 400 mg/kg of alcohpossesolic extract Ocimum sanctum flowers respectively.
Streptozotocin causes a massive reduction in insulin release by the destruction of the β-cells of the islets of Langerhans. An insufficient release of insulin, that leads to high blood glucose namely hyperglycemia. Insulin deficiency leads to various metabolic alterations in animals, such as increased blood glucose, increased cholesterol and transaminases. The fundamental mechanism is hyperglycemia that is over production of insulin and decreased utilization of glucose by the tissues.
The activity exhibited was compared with the standard antidiabetic drug (Glibenclamide). Daily treatment with the test extracts for a period of 21 days showed a significant decrease in serum glucose levels at 7th, 14th, 21st days and maximum reduction occurred at 21st day in diabetic rats. During the 21-day experimental period the body weight was reduced in diabetic rats, whereas as there was a significant gain of body weight in treated rats. The possible mechanism includes the stimulation of β-cells and subsequent release of insulin and activation of insulin receptor. Estimation of insulin level and insulin receptor may give more insight into the mechanism of the antidiabetic activity exhibited by the extracts.
Alcoholic extract of Ocimum sanctum leaves and flowers have beneficial effects on serum glucose levels and other metabolic aberrations as it lowers blood glucose level in diabetic rats. Therefore, Ocimum sanctum is considered to be an effective and alternative treatment for diabetes. Furthermore, these results suggest that both the leaves and flowers extracts contain antidiabetic active compounds, which would reduce the sugar level in STZdiabetic rats. Therefore, leaves and flowers extract could be used as hypoglycemic agents in diabetes management.
Medicinal plants have been using in traditional medicine to treat various diseases throughout the world over many centuries. Ocimum sanctum (known as Tulsi) is one of the most important medicinal plants which, serve medicine of various diseases. Different parts of Ocimum sanctum, such as leaves, stem, root, flowers and even whole plant have been used to treat fever, diarrhea, bronchitis, dysentery, asthma, etc. Tulsi contains Eugenol (1-hydroxy-2- methoxy-4-allylbenzene) active compound, which has been proven its therapeutic potential against diabetic, fungal and microbial infections. Ocimum sanctum also showed hepatoprotective, and cardioprotective properties. From nutritional analysis it is found that Ocimum sanctum leaves and flowers contain several important nutrients like sugar, protein, vitamin C, vitamin B1, vitamin B2, crude fiber, phenolic compounds and some essential minerals which are important for public health. This study provides a first step toward the development of nutritional standards for Ocimum sanctum in Bangladesh.
In this study, leaves and flowers of Ocimum sanctum showed activity against both gram negative bacteria and pathogenic fungi. Both leaves and flowers showed significant weight gains and remarkable reduction of blood glucose level in Steptozotocin-induced diabetic rats. The leaves and flowers of Ocimum sanctum could be used in traditional medicine system for the treatment of different types of disease. Therefore, further studies are needed to isolate active compounds which can be processed into new and potent medicines and to understand their mechanisms of action.
Author Contributions
All authors substantially contributed to the conception and design of the work. Finally, all authors discussed the results and contributed to the final manuscript.
All authors do not have any conflict of interests.
Citation: Siddique MAH, Maya SF, Sharma SCD, Roy N, Rahman MH, Shovon MS (2021) A Comparative Study on Nutritional Composition and Biological Activities of the Leaf and Flower of Ocimum sanctum Linn. J Nutr Food Sci. 11:816.
Received: 27-Jul-2021 Accepted: 10-Aug-2021 Published: 17-Aug-2021 , DOI: 10.35248/2155-9600.21.s6.1000816
Copyright: © 2021 Siddique MAH, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.