Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Review Article - (2017) Volume 5, Issue 4
Donkey’s milk is an interesting commercial product, due to its high nutritional value, and can be considered as a suitable source of food for human consumption. For the first time, it was performed the optimization study of clotting process using donkey milk and a plant aspartic protease-cyprosin, obtained from Cynara cardunculus flowers. An experimental design was used for the preliminary factors selection such as: the clotting enzymes (cyprosin and microbial rennet), CaCl2 concentration (0.01%-0.1% (w/v)) and clotting temperature (32-35ºC). The cyprosin and the 0.1% (w/v) CaCl2 concentration, as well as the relation between the CaCl2 concentration and the temperature, were the factors that showed a significant influence on the clotting activity. The 0.1% (w/v) CaCl2 concentration is an important factor because allows to obtain a more consistent curd, while the clotting activity of cyprosin was more efficient than microbial rennet. This preliminary study can give a new research base to obtain different types of cheeses using other milk origin as well as different clotting enzymes such as the cyprosin.
Keywords: Cheese making; Cyprosin; Donkey’s milk; Microbial rennet
Nowadays cow’s milk is widely used as a suitable substitute for the human milk but, in an increasing number of cases, it can lead to an abnormal immunological response. Consequently, cow’s milk protein allergy is the most common food allergy, affecting about 3% of children in the first three years of life [1]. To overtake this situation, donkey’s milk is a valuable product and it can be used in multiple applications, to manufacture dairy products as well as cosmetics, pharmaceutical active biomolecules and soaps. One of the main features of donkey milk besides on its resemblance to human milk, with similar lactose and mineral contents, fatty acid and protein profiles, which make it the most appropriate mammalian milk for infant consumption [2,3], and in those cases in which other milk types cannot be employed, such as in presence of cow’s milk allergies in children and adults [4,5]. Equine milk, comparatively with bovine milk, contains less fat, protein, inorganic salts, but more lactose, being close to human milk, as well as, it is considered to be highly digestible, palatable and rich in essential nutrients [6]. Its biological functions are also due to a high concentration of polyunsaturated fatty acids, low cholesterol content, high levels of vitamins A, B and C [7]. The protein composition is significantly different from cow’s milk: the total content is lower (1.5-1.8 g/100 g) and quite similar to that of human and mare milk; this condition avoids an excessive renal load of solute [8]. Donkey’s milk is considered as a suitable candidate as substitution of human milk for clinical tolerability, palatability and nutritional adequacy for children affected by a cow's milk protein allergy, furnishing additional physiological functions as well, such as providing antibacterial substances, digestive activity molecules, growth factors and hormones [9]. Donkeys’ milk is also is receiving increasing attention due to other interesting biological activities, such as the antioxidant activity [10], the immuno-stimulating ability and antiinflammatory effects, which may be useful in the treatment of immune-related diseases in humans and prevent atherosclerosis [11]. Moreover, other interesting activities have been reported, such as the antimicrobial properties, due to the high concentration of lysozyme and lactoferrin [12], the antiviral activity [13], and the antiproliferative effect on specific human lung cancer cells [14].
One of the problems associated with using of donkey’s milk is the processing difficulty in terms of other milk derived products such as the cheese, once the yield and poor clotting activity from different clotting enzymes is reduced. A decisive step in the possibility to use donkey’s milk in the dairy sector, for cheese production, has been made recently, thanks to the surprising finding of the Italian food technologist, which has discovered that pure camel chymosin, enzyme found in camel rennet, is able to clot effectively donkey’s milk [15]. Coagulating enzymes are an absolute necessity for the production of ripened cheese varieties. Vegetable enzymes, extracted from higher plant organs, have been extensively investigated as potential coagulants in cheese making. Cynara cardunculus L. flowers extract has been used for centuries in traditional artisanal production of ewe’s milk cheeses such as Serra da Estrela, Manchego, La Serena or Serpa in Portugal and Spain [16-18]. Proteolysis of cheeses processed with plant coagulants is more pronounced, leading to a soft and buttery cheese texture and, partly, to liquefaction and shape loss [16,19-22]. Another advantage to use protease from plant origin is related to bioactive peptides that are generated from casein proteolysis by proteases of C. cardunculus L. [23]. Cyprosins are three aspartic proteases originally denoted as cynarase 1-3 have been identified in extracts of C. cardunculus L. are heterodimeric enzymes with a molecular weight of 49 kDa, formed by a large subunit (32.0-34.0 kDa) and a small subunit (14.0-18.0 kDa) containing high mannose-type glycosylations [24,25].
Due to the high potential for industrial applications, the aim of this study was to show, for the first time, the clotting activity of the cyprosin for cheese production using donkey’s milk. This result is a significant advance toward to get an innovative, more nutritional and less allergenic product, being suitable to consumers that cannot tolerate other types of milk characterized by high allergenic reactions. This preliminary study showed also that cyprosin is more efficient than microbial rennet being a suitable alternative for production of cheese using donkey’s milk with different properties and nutritional advantages.
Experimental design
A two-factor factorial design is an experimental tool in which the data are collected for all possible combinations of the levels of the two factors of interest, being denoted using the exponential notation 2k, which compactly expresses that k factors with two-levels each are crossed, resulting in 2k experimental conditions (Box 2005) [26]. The design enables estimation of seven effects: three main effects, three two-way interactions, and a single three-way interaction. In this preliminary assay, the experimental design was based on two-level factorial design with aims to increase the clotting yield. It was studied three parameters such as: A-the clotting enzymes (cyprosin and microbial rennet from R. miehei ); B-CaCl2 concentration [0.1 (w/ v)-0.01% (w/v)] and; C-Temperature (32-35°C) (Table 1). Design Expert 10 software.
| Run number | Enzyme | CaCl2 (%) w/v | Temperature (°C) | Clotting Strength (U) | Average Clotting Strength (U) | Standard Deviation | |
|---|---|---|---|---|---|---|---|
| 1 | Cyprosin (-1) | 0.01 (-1) | 32 (-1) | 235.3 | 238.1 | 236.7 | ± 1.4 |
| 2 | Microbial rennet (+1) | 0.01 (-1) | 32 (-1) | 166.7 | 169.5 | 168.1 | ± 1.4 |
| 3 | Cyprosin (-1) | 0.1 (+1) | 32 (-1) | 333.3 | 444.4 | 388.9 | ± 55.6 |
| 4 | Microbial rennet (+1) | 0.1 (+1) | 32 (-1) | 266.7 | 259.7 | 263.2 | ± 3.5 |
| 5 | Cyprosin (-1) | 0.01 (-1) | 35 (+1) | 277.8 | 285.7 | 281.7 | ± 4.0 |
| 6 | Microbial rennet (+1) | 0.01 (-1) | 35 (+1) | 181.8 | 178.6 | 180.2 | ± 1.6 |
| 7 | Cyprosin (-1) | 0.1 (+1) | 35 (+1) | 298.5 | 307.7 | 303.1 | ± 4.6 |
| 8 | Microbial rennet (+1) | 0.1 (+1) | 35 (+1) | 250 | 256.4 | 253.2 | ± 3.2 |
Table 1: Code and parameters used in experimental design. The results of clotting activity using donkey milk by cyprosin were obtained in duplicated.
Extraction and purification of aspartic proteases
Fresh flowers (150 g) of the Cynara cardunculus were ground under liquid nitrogen to a fine powder. The ground tissues were homogenized in 50 ml Tris/HCl, pH 8.3, with 1 mM EDTA (TE buffer) containing 10%(w/v) poly (vinylpolypyrrolidone), at 4ºC for 2 h. The homogenate was centrifuged at 11,000 × g for 15 min at 4ºC, yielding the crude extract. The extract was dialyzed overnight against TE buffer at 4ºC. The enzyme purification involved loading the dialyzed enzyme extract into the loop and the transfer of the extract to an anionic exchange DEAE-Sepharose column (Pharmacia, Uppsala, Sweden) previously equilibrated with TE buffer and washed with the same buffer (90 ml) at a flow rate of 1 ml/min. Elution was performed using three gradients of 1 M NaCl in TE buffer: 0%-50% (10 ml), 50% to 100% (60 ml) and 100% (10 ml) at a flow rate of 1 ml/min. The pool of fractions (15 ml) with highest proteolytic activity was dialyzed, loaded into a second anionic exchange Q-Sepharose column (Pharmacia) previously equilibrated with TE buffer and washed with the same buffer (50 ml). The elution was performed with three gradients of 1M NaCl in TE buffer: 0-50% (20 ml), 50-100% (40 ml), and 100% (10 ml). The chromatographic steps were monitored using on FPLC system (Pharmacia) [25].
Enzymes preparation
The purified cyprosin and the commercial microbial rennet (Hannilase) produced by fermentation using the R. miehei strain were previously prepared according to the experimental conditions. Pure microbial enzyme (0.5 g) was dissolved in 25 ml of 10 mM sodium phosphate buffer, pH 7.4, at room temperature. Both enzymatic solutions presented the similar enzymatic activity (25 IMCU.ml-1) (IMCU-International Milk-Clotting Unit).
Milk and preliminary clotting assay
Donkey’s milk, obtained from a farm near Lisbon, Portugal, was previously pasteurized. The milk was warm at 65ºC during 30 min. After that, the calcium chloride was added according to the final concentration of 0.01% (w/v) and 0.1% (w/v). The factorial design experiments were performed using donkey’s milk samples (50 ml), pH 7.4 with the suitable CaCl2 concentration (Table 1). After a previous incubation at each specific temperature in water bath (32ºC and 35ºC), the proteolytic enzymes solutions (100 μl) were added. The milk clotting activity (U) was determined using the equation 1. The milk clotting duplicate assays were performed. The clotting activity or rennet strength was evaluated according to the method described by Chazarra et al. [27], with slight modifications. The temperature of the milk sample was controlled using a water-bath and the coagulation time was determined by checking visual gel formation at short time intervals. The rennet strength (RS) was defined as the fraction of volume of coagulated milk per volume of rennet in 40 min at 32°C and at 35°C. The RS is thus given by:
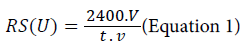
where v corresponds to the volume of clotting enzyme (ml), V is the volume of donkey’s milk (ml) and t corresponds to the rennet coagulation time (s).
Cheese assay
The donkey’s milk was previously pasteurized and the pH was adjusted to 7.4. Then, the CaCl2 was added to a final concentration [0.1% (w/v)]. The sample of milk was incubated at 32ºC, during 30 minutes. After that, 8 ml of purified plant enzyme were added. After 45 minutes the curds was formed and the whey was eliminated. The curds then formed were transferred to the suitable box for repining at 4ºC during 48 hours with salt.
The cyprosin has been used largely in the production of the different types of cheese with specific characteristics [18]. For a preliminary study, an experimental design was established to select the factors that have a strong influence in donkey’s milk coagulation, allowing evaluating the suitable experimental conditions to obtain a curd with a strong consistence. The aim of this study was to obtain a strong curd structures once the casein contents in the donkey milk is very poor. Then, for that reason, the pH was not considered as a key factor and the pH of the milk (7.4) was kept constant, considered the suitable value for donkey milk optimal value.
For an effective approach for experimental procedure, a multivariate statistical experimental design was used in which the factors of interest were changed simultaneously. The analysis of variance (ANOVA) was used to identify the factors that affect the donkey’s milk coagulation activity (Table 2). Based on Pareto chart, and according to the straight line in the bar plot the enzyme (cyprosin), the CaCl2 concentration and the interaction (CaCl2 vs temperature) showed to have a strong influence over the clotting activity, while the temperature did not show to have a significant influence over the final product (Figure 1, Table 1).
| Source | Sum of Squares | df | Mean Square | F Value | p-value Prob>F |
|---|---|---|---|---|---|
| Model | 32 438.645 | 3 | 10 812.88 | 22.75 | 0.0056 |
| A-Enzyme | 14 938.56 | 1 | 14 938.56 | 31.43 | 0.005 |
| B-CaCl2 | 14 577.78 | 1 | 14 577.78 | 30.67 | 0.0052 |
| BC-CaCl2-Temperature | 2922.3 | 1 | 2922.3 | 6.15 | 0.0682 |
| Residual | 1901.3 | 4 | 3475.33 | ||
| Total | 34 339.95 | 7 |
p-value are statistically significant at 95% of confidence level.
Table 2: Analysis of variance for clotting activity along the experimental assay.
According to the analysis of the factorial design results, the cyprosin and the CaCl2 concentration [0.1% (w/v)] as well as the interaction effect (BC), (CaCl2 concentration and temperature) play an important role in the clotting of the milk and curd consistency (Figure 2). The clotting yield of the microbial enzyme, based in both the clotting activity (U) and consequently in clotting time, was lower than cyprosin as it can be observed by the several assays performed. Then, the clotting activity was higher for assays that used plant aspartic protease, showing that this enzyme is more suitable for clotting process (Table 1). Additionally, the clotting yield using the microbial enzymes was lower than plant enzyme such as it was observed in terms of the volume of curd obtained along the assay (Figure 2). The enzymatic proteolysis of milk by plant aspartic proteases and by other aspartic proteases occurs by the hydrolysis of the phenylalanine-methionine bond of bovine k-casein, specifically between the amino acids Phe105- Met106 [25]. The proteolysis of plant enzymes over casein sequence is different comparatively to other types of aspartic proteases, such as animal or microbial sources. Then, the cyprosin is characterized by a high and specific clotting activity in cow, goat, ewe and buffalo’s milk. For that reason, the difference of curd produced is related to the type of aspartic proteases being an important factor in this study (Figure 2). The amount of the whey is significant comparatively with others types of milk, once the donkey’s milk, presents a specific composition such as the high lactose concentration (7.4%), similar to human milk (7.0%), but low concentration in terms of protein (2.0%) and fat (1.4%) [28].
The positive effect of the enzyme and the temperature is related to the suitable experimental conditions characterized by lower coded values (cyprosin and 32ºC), while the factor B (CaCl2 concentration) showed significant effect in the top coded value [0.1% (w/v)]. The aspartic proteases from C. cardunculus plant presents also huge advantages associated to some products obtained from casein degradation in cheeses as well as higher concentration of hydrophobic peptides [29,30]. Studies carried out by Macedo et al. [31] showed also that the aspartic proteases from C. cardunculus flowers produce the same macropeptide fragment from -casein as calf chymosin. The CaCl2 concentration is the second factor with highest influence in clotting activity. The concentration [0.1% (w/v)] allowed increasing the clotting activity and the presence of calcium ions helped the curd formation and consequent firmness increasing, reducing the susceptibility to break curd during the removal of whey in the initial phase of cutting step. The interaction between the CaCl2 concentration and the temperature is important, because the clotting activity is high when the CaCl2 represented by high values 0.1% (w/v) and temperature is characterized by its low value (32ºC), respectively. The statistical analysis showed that the model presented a standard deviation of 21.80 and a R2=0.95, and based on F-value=22.75 the model is significant (Table 2). The predicted R2=0.78 is reasonable in agreement with adjusted R2=0.90 due to the small difference that occurs. The following coded equation, based on the experimental data, can be used to predict the response for a specific level of each factor, allowing to identify the relative impact of each factor coefficients (Equation 2).
R = 259.41 ? 43.21 × Enzyme + 42.68 × CaCl2 ? 19.11 × CaCl2 × Temperature (Equation 2).
This preliminary study allowed to define which the factors analyzed present the most influence in clotting activity, once it allowed to reached theoretically the rennet strength 380 U, using the cyprosin, 0.1% (w/v) CaCl2 at 32ºC. It is possible to refer that the donkey milk presented a low yield of curd because the proteins contents is low comparatively with other species such as the cow, sheep or goat (Figure 2). After that, a cheese of donkey’s milk was produced presenting a suitable consistency, after 5 days in the refrigerator at 4ºC for ripening (Figure 2). Comparatively to other types of milk such as cow milk, 5 liters allowed to produce a 700 g of cheese of (14%), while for donkey milk the yield is about 6.25% due to its composition. Donkey’s milk coagulation is extremely difficult and the product yield is reduced due to its low concentration of caseins (2.0%) comparatively with other species milk such as cow (2.8%); goat (2.5%) or sheep (4.6%) [28]. According to the study performed by Steinsholt and Ystgaard [32] there is a positive correlation between the yield of cheese and the casein fraction, fat content and total solids of milk. Relatively to the syneresis, it was easy to remove the huge volume of whey, because the donkey milk presents a low percentage of caseins and other proteins.
Then, based on this preliminary study, it was possible to select the suitable conditions to obtain the high clotting activity and consequently to produce a cheese using donkey milk and an aspartic protease from plant origin. The products obtained can be suitable for consumers that present allergic reaction relatively to other types of milk, being also an option for new and rich nutraceuticals products.
This study was supported by Faculty of Engineering, Lusophone University of Humanities and Technologies, facilities and laboratory staff.
Compliance with ethical standards.
Author declares that they have no competing interests.