Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Commentary - (2015) Volume 3, Issue 2
Background: The microbial load of milk is a major factor in determining its quality, which is indicates the cleanliness of the milking utensils, condition of storage, manner of transport as well as the cleanliness of the udder of the individual animal. The aim of this study was to determine microbial quality of cow’s milk.
Methodology: The study was conducted in Dawa Chefa District, Oromia Zone of the Amhara National regional state. A total of 50 samples of raw cow's milk were collected at morning from hotels (27 samples), cooperative (3 samples) and household producers (20 samples). The farmers (producers) that involve in the study were selected based on potential of milk production, market orientation and willingness of households. Samples of raw morning milk were taken from each sampling point twice in a month. During collection, approximately 250 ml raw milk sample were taken aseptically from the owner’s container then placed into sterile glass bottles.
Result: The average total plate counts of milk sampled from farmers, dairy cooperatives and hotels were 6.88 log10, 7.10 log10 and 7.54 log10, respectively. The average coliform counts were 5.57 log10 at farm level, 5.63 log10 for dairy cooperative and 5.37 log10 for hotels. The average yeast and mould counts at farmer, dairy cooperative and hotel were 0.46 log10, 0.62 log10, and 0.74 log10 cfu/ml, respectively. The indirect tests and the actual bacterial count indicated that the microbial quality of milk produced by farmer and collected by dairy cooperatives in the study area was poor and this calls for scrupulous hygienic measure during production and handling of milk.
Conclusion: The result showed that the microbial quality of raw milk obtained from local dairy farmer was very low. This was due to the unhygienic condition of milking; unclean milk handling equipment and the use of contaminated water. Therefore, Proper hygienic practice will become a useful parameter and model for other dairy farmers within the district to improve and upgrade their dairy production.
Keywords: Coliform, Microbial, Milk, Quality, Yeast
Ethiopia is believed to have the largest livestock population in Africa estimated about 53.4 million heads of cattle [1] of which 10.5 million is dairy cows. Dairy production, among the sector of livestock production, is a critical issue in Ethiopia where livestock and its products are important sources of food and income, and dairying has not been fully exploited and promoted in the country [2]. Despite its huge dairy cattle population of Ethiopia, the per capita milk consumption in Ethiopia 19 litters is very low as compared to the global average of 100 litters and even far below the average of Africa, 27 kg/year. The dairy industry in the country is constrained by several technical and economic factors (genetic limitation for production, inadequate and poor quality of feed resources, and prohibitive price of crossbred heifer’s and prevalent animal diseases) [3].
The microbial load of milk is a major factor in determining its quality. It indicates the hygienic level exercised during milking, that is, cleanliness of the milking utensils, condition of storage, manner of transport as well as the cleanliness of the udder of the individual animal [4,5]. Higher bacterial contents exist in developing countries where production of milk and various dairy products takes place under rather unsanitary conditions and poor production [6]. This implies, high numbers of bacteria in raw milk usually indicate heavy contamination caused by handling, inadequate cooling or both.
Milk and milk products represent an important place in the nutrition of consumers as well as nutrition and income of producers, there is limited work so far undertaken regarding assessment of hygienic practices and microbiological quality of raw milk in northern Ethiopia in general and in Dawa Chefa District in particular. Moreover research activities conducted in this area have been very much limited, and no researches have been done to identify the microbiology of milk in Dewa Chefa of the Amhara Regional State. Therefore, the current study aimed to determine the microbial quality of cow milk in the Dawa Cheefa District.
Description of study area
The study was conducted in Dawa Cheefa District, Oromia Zone of the Amhara Regional State. The area is located at 10°43′N, latitude and 39°52′E longitude. The altitude of the area ranges from 1500 to 2300 meter above sea level. The study area falls within the dry Kolla agro-climatic zone, which can be classified as semi-arid climate according to climatic classification developed in the Agro-ecological Zones of Ethiopia. The rainfall distribution of the study area has highly seasonal and temporal variations. There are two main rainy seasons in a year i.e. short rainy season (February to March) and main rainy season (July to September). The predominant production system in this area is mixed crop livestock farming. Cattles are the most important livestock species in the area (Figure 1).
Study plan
The laboratory experiments was conducted to assess microbial quality of milk by using microbial tests of standard plate count, coliform count and yeast and mould count (YMC).
Milk sample collection
A total of 50 samples of raw cow's milk were collected at morning from hotels (27 samples), cooperative (3 samples) and household producers (20 samples). The farmers (producers) that involve in the study were selected based on potential of milk production, market orientation and willingness of households. Samples of raw morning milk were taken from each sampling point twice in a month. During collection, approximately 250 ml raw milk sample were taken aseptically from the owner’s container then placed into sterile glass bottles. Consequently, samples were labeled and put in icebox (4°C) to restrict microbial multiplication and transported as early as possible to the ANRS Livestock Resources Development Promotion Agency Kombolcha Animal Disease Survey Investigation Diagnostic laboratory, which is 51 km far from the study area to analyze microbial quality.
Standard plate count
For total plate count, appropriate decimal dilutions was selected that would give the expected total number of colonies on a plate, i.e., between 30 and 300 colonies [7]. The standard plate count (SPC) agar was cooled to 45°C after autoclaved and before pouring to petri-dish. One ml of milk sample was added into sterile test tube containing nine ml peptone water up to serial dilution of 10-7 and mixed thoroughly. Then one ml of the sample from appropriate decimal dilution was placed on a petri-dish and then molten agar medium (10-15 ml) was poured onto the petri-dish and then it was incubated for 48 hours at 32°C. Finally, colony count was made using colony counter. The estimated number of colonies was calculated by:
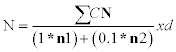
Where, N=Number of colonies per ml of milk sample; ∑C=Sum of all colonies on plates counted; n1=Number of plates used in lowest dilution counted; n2=Number of plates used in highest dilution counted; d=dilution factor of the lowest dilution used.
Coliform count
A serial decimal dilution was prepared using 0.1% peptone water. Duplicate appropriate decimal dilutions was surface plated and incubated at 32°C for 24 hours on Violet Red Bile Agar and typical dark red colonies on uncrowned plates were considered as coliforms and counted. This was followed by a confirmatory test by transferring and incubating four to five typical colonies from each plate transferred into tubes containing 2% Brilliant Green Lactose Bile. Gas production within 48 hours of incubation at 35°C was considered as sufficient evidence for the presence of coliforms [7].
Yeast and mould count
Samples of milk were serially diluted in peptone water and volumes of 0.1 milliliter of appropriate dilutions were plated in duplicate. Petri-dishes by the pour plate techniques using chloromophenicol agar was used. The agar consisting of 5 g yeast extract, 20 g glucose, 0.1 g chloramphenicol, 0.01 g bromophenicol blue, and 15 g agar per liter of distilled water at a pH of 6.0 to 6.2. The dried plates were then incubated at 25°C for 3 to 5 days. Colonies with a blue green color was counted as yeasts and moulds [8].
Data management and statistical analysis
The microbial count data were first transformed to logarithmic values (log10) before statistical analysis. Then, data on the quality and the transformed microbial count values were analyzed using the General Linear Model (GLM) procedure of Statistical Analysis System [9]. Mean separation was carried out using the Least Significant Difference (LSD) technique when analysis of variance shows significant differences between means. The following model was used for the milk microbial count data:
Yij = μ + βj + eij
Where, Yij=individual observation for each test; μ=the overall mean; βj=the ith milk source effect (producer, hotels and cooperatives); eij=the error term
Microbial quality of cow’s milk
Standard plate count: The current study result shows that, there was no significant difference (P?0.05) in bacterial load among the three milk sources (Table 1). The bacteriological quality for most raw milk sample collected from hotels was poor with a total plate count of 7.54 log10 cfu/ml).The overall mean of total plate count of 7.25 log10 cfu/ml in this study was higher than those found in the study of Zelalem [10] (6.46 log10 cfu/ml) who mentioned that, improper hygienic practices during milking process, poor storage temperature, health and hygiene of cows and procedures used in cleaning and sanitizing the milking and storage equipment to affect the microbiological quality of raw milk. In addition to this ineffective sanitizing routine, the cow’s environment that leaves manure in contact with cows’ udder also contributed to the high bacterial load in raw milk. Another finding conducted by Asaminew [11] reported 7.58 log10 cfu/ml higher total plate counts as compared to the current study. The average total plate count found in the present study was higher than the acceptable value by American Public Health Association (1992) which is 2 ×105-4×105 cfu/ml. The current result was also beyond the range of total bacterial count of developing countries, which is 5.301 to 5.875 log10 cfu/ml [12].
| Source | TPC | CC | YMC |
|---|---|---|---|
| Hotel | 7.54 ± 0.26 | 5.37 ± 0.19 | 0.74 ± 0.03a |
| Farmer | 6.88 ± 0.31 | 5.57 ± 0.22 | 0.46 ± 0.035b |
| Cooperative | 7.10 ± 0.79 | 5.63 ± 0.56 | 0.62 ± 0.09ab |
| Over all mean | 7.25 | 5.47 | 0.622 |
Table 1: Average microbial count (log10 cfu/ml) obtained from individual farmer, dairy cooperative and hotels (values are mean ± SE).
a,bMeans in a column with different superscripts differ (P<0.05); TPC: Total Plate Count; CC: Coliform Count; YMC: Yeast and Mould Count.
Coliform count
Analysis of variance suggested that lack of difference (P?0.05) in coliform counts of the three milk sources (Table 1). The coliform count result of this study were higher compared to the coliform count of 4.18 log10 cfu/ml noted by Abebe et al. [13] in Ezha district of Gurage zone, the 4.49 log10 cfu/ml by Asaminew [14] in West Shewa Zone of Oromia Region and the 4.84 log10 cfu /ml noted by Derese [15] for Bahir Dar milk shed. The overall value of coliform count observed in this study was much higher than the recommended value given by American Public Health Service and was greater than 100 cfu/ml for grade A milk, and 101-200 cfu/ml for grade B milk [16]. Generally all milk samples in this study had higher than the recommended level (102 cfu/ml). This might be attributed to the hygienic condition such as dirty equipment, contact with manure of the cow during milking and personal hygiene of the milkers. Generally the presence of high number of coliform in milk indicates that the milk has been contaminated with faecal material and it is an index of hygienic standard used in the production of milk.
Yeast and mould count
Mean value of yeast and mould count were significantly different (P<0.05) among the different sample sources within the same district, and was greater in milk samples obtained from hotels as compared to milk sampled from the producers . The mean value yeast and mould found in the current study at farmer (0.46 log10), hotel (0.74 log10 cfu/ml) and cooperatives (0.62 log10) was below the recommended level of yeast and mould given by the Malaysia food quality standard of yeast and mould in raw milk sample, which should be lower than 2.1 log cfu/ml [17].
The result showed that the microbial quality of raw milk obtained from local dairy farmer was very low. And this was due to the unhygienic condition of milking; unclean milk handling equipment and the use of contaminated water were among the important source of milk contamination. High bacterial load, the presence of pathogenic bacteria in several samples not only affects the raw milk quality but definitely pose a safety issue to consumer. The introduction of proper training and hygiene practice during milking to the dairy farmer was found to be efficient in reducing the bacterial load or contamination of raw milk.
Competing interest: The authors declare that they have no competing interest.
SA conceived the study, designed and conducted all laboratory experiments; analyzed and interpreted experimental results. ME, GA and KG participated in the proposal, study design and manuscript preparations. All authors read and approved the final manuscript.
I am deeply grateful and indebted to Amhara National Regional State of livestock Resources Development Promotion Agency, Kombolcha Animal Disease Survey Investigation Diagnostic Laboratory that support me by creating fertile ground to laboratory work.