Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2019)Volume 7, Issue 2
Yoghurt is considered as the best and the most complete food, but it is deficient in iron and by fortification yoghurt iron can reach for most consumers. Four different iron salts were used (iron amino acid chelate (T1), ferrous sulfate (T2), ferrous fumarate (T3), and ferric hydroxide poly maltose (T4)) for fortification of such yoghurt. Chemical properties, acidity, lactic acid bacteria, yeasts, rheology, viscosity, peroxide number and sensory properties were evaluated at zero time, after 3 days and after 7 days of manufacturing. Total solids were increased by iron salts, especially in T1 and T4 treatments followed by T3 and the T2. The T2 was the highest treatment in ash content, while the protein content was higher in T1 and the T4 in contrary to protein contents in T2 and T3 that did not affect by adding iron salts. In all treatments, fat contents did not affect by the addition of iron. The T1 and T3 had the lowest acidity, while theT2 and T4 were the highest however, the acidity in the control lie between them. The peroxide numbers for yoghurt belongs to different treatments were estimated after the cold storage for 7 days and ranged between 0.65 meq O2/kg in T4 and 0.98 meq O2/kg in the T2. The peroxide number in T1, T3 and T4 were lower than the peroxide number in the control. All different samples are accepted by panelists. In conclusion, iron can be added to yogurt in different forms without affecting the characteristics of resultant yoghurt with preference the 1st treatment (i.e. yoghurt Fe fortified by amino acid chelate).
Yoghurt; Iron salts; Chemical properties; Peroxide number; Microbiological test; Viscosity; Sensory evaluation
One of essential micronutrient in human nutrition is iron. It is also a component of heme in hemoglobin and myoglobin in which it plays important role in the transport, storage and utilization of oxygen.
Iron deficiency induces anemia, alters mental development, decreases immunity impairs cognitive scores in children and leads to poor pregnancy outcome and lowers working capacity in adults [1]. Iron in food may be highly bioavailable as is the case in the iron found in the heme which is found in red meat, but the cost of these products may be high for many people. The iron present in other products of vegetable origin, is non-heme and has the disadvantage of interacting with substances in food that inhibit its absorption such as tannins, phytates, and polyphenols hence it has low bioavailability. Much of this kind of food is consumed by people in the lower socioeconomic classes, who thus cannot meet their physiological needs for iron [2]. Therefore it is widespread in less industrialized countries as in developing countries. Iron deficiency is also caused by either insufficient dietary intake of iron, poor absorption of iron or both [3].
Dairy products are an important group in human nutrition. Direct addition of iron to dairy product might be effective way to increasing the dietary intake of iron to the general population. Yoghurt is excellent source of vitamins, minerals and proteins but its iron concentration is low (approximately 0.2 mg/kg [3] which makes it impossible to meet iron Recommended Daily Allowance (RDA). Therefore dairy products are logical carrier for iron fortification [4] and considered as practical and cost-effective long term solution [5].
Since fermented milk products are among highly-consumed food in the world, they have been used to deliver nutritional components into human diet. Furthermore, fortification of these products such as yoghurt is a good way to improve nutrient intake in daily food products [6]. Fortification of dairy products with Fe would help nutritional deficiencies. Iron-fortified yoghurt has a relatively high iron bioavailability [7]. However, before doing any process such as fortification, the effects of added iron to yoghurt must be assayed.
The parameters including oxidation of fat, taste, shelf life and microbial physiology are important, and the sensory quality and overall acceptance of fortified yoghurt must be ascertained [8]. Daily iron requirements for adults are 19.3-20.5 mg/day in men and 17.0-18.9 mg/day in women older than 19 in average 18.9 mg/day [9]. Bioavailability of different iron compounds used to fortify formulas is 30%. Therefore the purpose of the research was to provide about onethird of the daily needs of the adult iron by consuming yoghurt by adding 20 mg iron in the common serving quantity to give in consideration that vital availability (30%) is one-third of daily needs.
Materials
Cow's milk was obtained from the local market of Damanhour city, Behera Government, Egypt. Yoghurt culture was obtained from CHRHansen` s laboratories, Denmark, under commercial name type (FD– DVS–YC–X11) containing Streptococcus thermophilus and Lactobacillus delbrueckiissp . Bulgaricus. All the chemicals used were purchased from Sigma Chemical Company, USA. Media used for microbiology tests were: M17, MRS, MaCconkey broth and PDA obtained from Oxoid Ltd., Basingstoke, Hampshire, England.
Yoghurt making procedure
Cow´s milk was heated to 95°C/5 min, then divided into 5 portions each one 2.5 kg milk: The first portion (T1) was fortified with 75 mg iron amino acid chelate, the second portion (T2) was fortified with 160 mg ferrous sulfate, the third portion (T3) was fortified with 65 mg ferrous fumarate, the fourth portion (T4) was fortified with 65 mg ferric hydroxide poly maltose which all provide 20 mg Fe/Kg milk. The last portion (T5) was without iron add and regarded as control. All treatments were cooled to 45°C, inoculated with (3%) yoghurt culture and filled into 80 ml plastic cups and incubated at 42°C until a firm curd was formed. The resultant yoghurt was kept in a refrigerator (4°C ± 1°C) for a week.
Methods of analysis
Chemical analysis: The samples were analyzed for determination of total solids using dry oven at 105°C for 6 h as described in [10] AOAC. Protein, Fat, Titratable acidity, were determined according to Lin [11]. Viscosity was measured by a viscometer (Haakegeorzauee, Germany).
Microbiological tests: Streptococci Count was enumerated according to Tabasco [12] using M17 agar, Lactobacilli was enumerated as described by [11] Tabasco using MRS media at 42°C for 48 h, Coliform bacteria were determined (most probable number ) as described by APHA [13]. While molds and yeasts were enumerated on Potato Dextrose Agar (PDA) at 25°C for 5 days according to Frank [14].
Texture profile analysis (TPA) of yoghurt: Texture profile analysis (TPA) was done for yoghurt samples using the double compression test (Multi test 1d Mecmesin, Food Technology Corporation, Slinfold, W.Sussex, UK) at room temperature by compression test that generate plot of force (N) versus time (s). A 25 mm diameter perplex conical shaped probe was used to perform the TPA analysis of samples in five different points on the sample surface.
In the 1st stage, the samples were compressed by 30% of their original depth at a speed of 2 cm/min during the pre-test, compression and relaxation of the sample. From the force-time curve, the following parameters were determined according to the definition given by the International Dairy Federation [15] as follows:
Hardness (N)=maximum force of the 1st compression
Cohesiveness=area under the 2nd compression/area under the 1st compression (A2/A1)
Adhesiveness (N.s)=negative area in the curve (A3)
Springiness (mm)=length 2nd compression/length 1st compression (L2/L1)
Gumminess (N) g=Hardness × Cohesiveness
Chewiness (mJ) g/mm=Gumminess × Springiness
Viscosity: Yoghurt viscosity was measured according to Hamed [16]. The apparent viscosity of yoghurt was measured using a Bohlin coaxial cylinder viscometer (Bohlin Instrument Inc., Sweden) attached to a work station loaded with software V88 viscometery programmer.
The viscometer probe, system C30, was placed in the yoghurt samples cup, and measurements of viscosity were carried out at 20°C ± 2°C in the up mode at shear rate ranging from 37 to 1238 1/s.
Determination of Peroxide number: Acetic Acid-Chloroform Method
To determine the peroxide number of fats, in terms of meq O2 per Kg of sample, The Official Method Cd 8-53 of the American Oil Chemists’ Society [17] was used.
Sensory evaluation: The sensory evaluation of products was carried out 1, 3 and 7 days after treatment. Each yoghurt sample of different treatments was evaluated for color, flavor, texture and allover acceptability using a hedonic scale from 9 to 1 (9=like extremely, 5=neither like nor dislike, 1=dislike extremely).
All samples were presented to the assessors at room temperature under normal lighting conditions in transparent glass cups coded with random, three- digit numbers [17]. The average value scores of all sensory evaluations were used in the analysis.
Statistical analysis: Statistical analysis was performed according to [18] SAS Institute using General Linear Model (GLM) with the main effect of addition ratios. Duncan's Multiple Range test was used to separate means among of three replicates at p<0.05 for chemical analysis and among panelists in the sensory evaluation tests.
Chemical analysis
Table 1 shows the chemical composition of yogurt resulting from all treatments in which iron is added in different forms compared to the control. It is clear that there were small differences, such as in % of the total solids and fat, at the same time significant differences between samples for each of ash and protein.
| Treatment | Fresh | 3 days | 7 days | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TS % | Ash% | Protein % | fat% | TS% | Ash % | Protein % | Fat% | TS% | Ash% | Protein % | Fat% | |
| T1 | A11.5a | A0.85a | A3.60a | A3.50a | A11.5a | A0.55b | A3.60a | A3.57a | A11.5 a | A0.58a | A3.60a | A3.60ab |
| T2 | A11.3a | A0.82b | A3.40bc | B3.44ab | A11.4ab | A0.82c | A3.40bc | AB3.49b | A11.4ab | A0.85bc | A3.30b | A3.51bc |
| T3 | A10.9a | A0.85a | A3.50ab | B3.50 a | A10.8b | A0.87ab | A3.50ab | A3.61a | A10.8b | A0.58ab | A3.60a | A3.64a |
| T4 | A11.5a | A0.55a | A3.30c | B3.30b | A11.6a | A0.55b | A3.30c | A3.39c | A11.6a | A0.54c | A3.20b | A3.42c |
| T5 | A10.9a | AB0.88c | B3.40b | B3.30b | A10.5b | B0.75d | B3.50ab | AB3.36c | A10.8b | A0.81d | A3.80a | A3.41c |
| T1: Iron amino acid chelate; T2: Ferrous sulfate; T3: Ferrous fumarate;T4: Ferric hydroxide poly maltose; T5: Control | ||||||||||||
| *Mean of triplicate determination followed by the same manuscript ( right small manuscript between column, left capital manuscript between row) are not significantly different at p ≤ 0.05. | ||||||||||||
Table 1: Chemical composition of different iron fortified yoghurt samples during storage at 4°C ± 1°C*.
These results are consistent with that was stated by [19,20] mentioned that the fat content seems to be not affected by adding iron to yoghurt. It is worth mentioning that Codex for fermented milk and yoghurt requires that the concentration of protein should not be less than 2.7% and fat must be below 10% [21].
Table 2 shows the % acidity in the yoghurt produced by adding different iron salts. The acidity at zero time was more or less the same and ranged between 0.975%-1.040% which showed that the addition of iron in different sources did not affect the acidity in the yogurt produced at zero time.
| Treatments | Fresh | 3 days | 7 days |
|---|---|---|---|
| T1 | B0.9754a | A1.2470b | A1.2660c |
| T2 | C1.0036a | B1.3400a | A1.4300a |
| T3 | C1.0400a | B1.3300a | A1.4900a |
| T4 | C0.8628a | B1.2600b | A1.3500b |
| T5 | B1.0030a | A1.1700c | A1.2260c |
| T1: Iron amino acid chelate; T2: Ferrous sulfate; T3: Ferrous fumarate;T4: Ferric hydroxide poly maltose; T5: Control | |||
| *Mean of triplicate determination followed by the same manuscript (right small manuscript between column, left capital manuscript between row) are not significantly different at p ≤ 0.05. | |||
Table 2: Acidity (%) of iron fortified yoghurt samples during storage at 4°C ± 1°C*.
But the type of added iron had a significant role in the rate of acidity increase during cold storage which showed increases in the acidity with different trends. The most pronounced and significant increases were observed for T2, T3 and T4 followed by T1. Generally, the control (T5) was the lowest acidity during storage up to 7 days at 4°C ± 1°C. It is worth mentioning that Codex for yoghurt requires a minimum concentration of titratable acidity of 0.6% [22].
Total lactic acid bacteria
Table 3 shows the numbers of Streptococcus and Lactobacillus in the yoghurt samples produced by addition of different iron salts, and it is clear that there is a significant difference in the proportion of the numbers of the Streptococcus and Lactobacillus . In all treatments - except the second treatment T2, the number of Streptococcus bacteria was significantly higher than that of the control as a direct effect of the addition of the iron that activated the microbial growth in the Fe fortified yogurt and this effect varied between the different treatments.
| Treatments | Streptococcus | Lactobacillus count | Molds and Yeasts |
|---|---|---|---|
|
count (CFU/ml) |
(CFU/ml) |
(CFU/ml) |
T1 |
30*107 |
12*103 |
- |
T2 |
35*106 |
15*103 |
- |
T3 |
10*107 |
19*103 |
- |
T4 |
40*107 |
62*103 |
- |
T5 |
65*106 |
35*103 |
- |
| T1: Iron amino acid chelate; T2: Ferrous sulfate; T3: Ferrous fumarate;T4: Ferric hydroxide poly maltose; T5: Control | |||
Table 3: Lactic acid bacteria and yeast and mold in cold stored iron fortified yoghurt samples* Storage at 4°C ± 1°C for 7 days.
Data for the titratable acidity confirm such results for the number of Streptococcus in Fe added yoghurt. In contrast to Streptococcus , the Lactobacillus number was slightly reduced compared to control except for the fourth treatment (T4, ferric hydroxide poly maltose), which showed an increase in the number of Lactobacillus . Moreover, Table 3 indicates that all treatments were free of yeast and mold. Sum of microorganisms constituting the starter culture (min 107 cfu/g, in total) and Labeled microorganisms (min 106 cfu/g, in total) with free of yeasts and molds were identified by Codex [22] for yoghurt.
Food rheology is the study of the deformation and flow of food materials [23]. Texture is one of the most important properties for yoghurt quality. Yoghurt can be classified as pseudoplastic material (contains a yield stress that has to be exceeded for flow to be initiated) that can be either a viscoelastic fluid if we are dealing with stirred or drinking yogurt or a viscoelastic solid if we are dealing with set yogurt.
Viscoelastic indicates the material has some of the elastic properties of an ideal solid and some of the flow properties of an ideal (viscous) liquid. Yogurt also exhibits time-dependent shear thinning behavior but yogurt is not a true thixotropic material since structural breakdown due to shear is not completely reversible once the shear stops [24].
Data for the texture profile of different yoghurt treatment at zero time (2nd day of processing) are shown in Table 4. It is clear that the forms of Fe affected markedly the hardness values. The hardness values generally ranged between 0.3 N (T1) and 0.7 N (T3). Despite the hardness value of T5 (control) was more or less the same with T1, T2 and T4, the hardness of T3 (ferrous fumarate) increased markedly (0.7 N).
| Treatment | Hardness (N) | Springiness (mm) | Cohesiveness | Gumminess (N) | Chewiness (N*mm) |
|---|---|---|---|---|---|
| T1 | 0.3 | 0.45567 | 0.462527 | 0.138758 | 0.063228 |
| T2 | 0.5 | 0.422764 | 0.423913 | 0.211957 | 0.089608 |
| T3 | 0.7 | 0.511759 | 0.457565 | 0.320295 | 0.163914 |
| T4 | 0.5 | 0.5158 | 0.564139 | 0.282069 | 0.145491 |
| T5 | 0.4 | 0.839506 | 0.789569 | 0.315828 | 0.265139 |
| T1: Iron amino acid chelate; T2: Ferrous sulfate; T3: Ferrous fumarate;T4: Ferric hydroxide poly maltose; T5: Control | |||||
Table 4: Texture profile analysis of different Fe- fortified yoghurt samples.
The springiness values showed that addition of Fe in different forms markedly decreased the springiness of all yoghurt samples compared to control. Different yoghurt samples fortified by Fe had springiness values ranged between 0.422764 m and 0.515800 m compared to the control (0.839506 m). These sharp declines in springiness values due to Fe addition still acceptance of different yoghurts by panelists.
The Cohesiveness values of different yoghurt samples affected markedly due to addition different forms of Fe since their corresponding values varied from 0.423913 ss (T3) to 0.564139 ss (T4) which markedly declined compared with the control (0.78956 ss).
The main effect of addition Fe on the gumminess character was observed when Fe added in the form of iron amino acid chelate since the markedly decline was observed in its value (0.138758 N) compared to the other yoghurt treatments and the control since their gumminess values ranged between 0.211957 (T2) and 0.320295 (T3).
The major rheology character affected by addition Fe in yoghurt was the chewiness (Nmm). The chewiness of control 0.265139 markedly declined by about 50% as in T3 and T4 and by about 75% as in T1 and T2.
In general, these rheological effects of adding Fe in yoghurt that seemed to be more pronounced but at the same time did not influence any marked deteriorative effect in the sensory evaluation of such yoghurts.
Apparent viscosity of yoghurt
Viscosity of yoghurt affected by the number and strength of bonds between casein micelles, also their structure and spatial distribution [25]. Figure 1 shows the effect of shear rate on yoghurt apparent viscosity. As can be seen, initially, the apparent viscosity of all samples did not significantly affect by an increment of shear rate. Then, the apparent viscosity of all samples drastically reduced with the increasing of shear rate. Yoghurt shows a variety of non-Newtonian behaviors, such as yield stress, shear-thinning, viscoelasticity and timedependency [18]. The apparent viscosity of all fortified yoghurts is higher than the control.
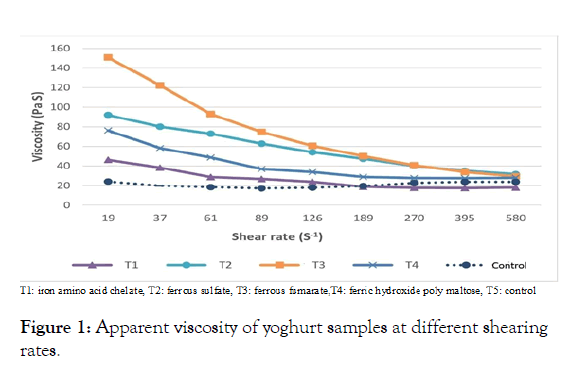
Figure 1: Apparent viscosity of yoghurt samples at different shearing rates.
Yoghurt is a gel system of casein micelles with entrapped water [26]. Adding salts may underpin gel structure of the fortified treatments, which indicates the consolidation of a portion of the relatively coherent gel structure, hence a higher bonding density per unit volume. Significant differences were recorded between the effect of different treatments on the apparent viscosity of the resultant yoghurt. T3 showed the highest value of apparent viscosity compared to all other the treatments followed by T2, T4, T1 and control treatment, respectively. This result can be explained that as a variation of the ionic strength of the salts used which affecting the gel matrix of yoghurt.
Peroxide number of yoghurt
Table 5 shows the peroxide numbers of all treatments after cold storage for 7 days. Values were between 0.65 meq. O2/Kg in the fourth treatment and 0.98 meq. O2/Kg in the second treatment, while the peroxide number in the first, third and fourth treatments was less than peroxide number in the control. However, it was clear that all samples had very low peroxide number (below 1 meq. O2/Kg).
| Treatment | Peroxide number (meq O2/kg) |
|---|---|
| T1 | 0.75 |
| T2 | 0.98 |
| T3 | 0.67 |
| T4 | 0.65 |
| T5 | 0.83 |
| T1: Iron amino acid chelate; T2: Ferrous sulfate; T3: Ferrous fumarate;T4: Ferric hydroxide poly maltose; T5: Control | |
Table 5: The peroxide number of iron fortified yoghurt after storage for 7 days at 4°C ± 1°C.
Sensory evaluation
The effect of different iron salts on the sensory evaluation of yoghurt during storage period at 4°C ± 1°C for 7 days are shown in Table 6. In fresh samples, the T2 treatment had the best color, flavor and texture, while its overall acceptance rated the lowest. The control treatment was evaluated the best for overall acceptability followed by T1 and T3. After 3 days, T1 treatment recorded the best value for all sensory attributes compared to other treatments. After 7 days, T1 treatment got the highest score for all sensory properties compared to other treatments.
| Treatment | Storage period | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 days | 3 days | 7 days | ||||||||||
| Color | Flavor | Texture | Allover Acceptability | color | Flavor | Texture | Allover acceptability | Color | Flavor | Texture | Allover acceptability | |
| T 1 | A7.75 ± 0.47c | B8.00 ± 0.05c | A8.50 ± 0.28a | A8.50 ± 0.28b | A7.75 ± 0.25a | A8.25 ± 0.47a | B8.00 ± 0.41a | A8.50 ± 0.50a | B7.50 ± 0.28b | C7.75 ± 0.25b | C7.50 ± 0.28a | B7.50 ± 0.28a |
| T 2 | A8.75 ± 0.47a | A8.50 ± 0.28a | A8.50 ± 0.25a | A8.25 ± 0.25c | B7.75 ± 0.25a | B8.00 ± 0.41b | B7.75 ± 0.47b | A8.25 ± 0.25b | C7.50 ± 0.28b | B7.75 ± 0.25b | C7.25 ± 0.25b | B7.25 ± 0.47b |
| T 3 | A8.50 ± 0.28b | A8.25 ± 0.47b | A8.00 ± 0.05c | A8.50 ± 0.28b | C7.25 ± 0.25b | C7.50 ± 0.28a | A8.00 ± 0.41a | B8.00 ± 0.41c | B7.75 ± 0.25a | B8.00 ± 0.05a | B7.50 ± 0.28a | C7.50 ± 0.28a |
| T 4 | A7.75 ± 0.62c | A8.50 ± 0.28a | A8.25 ± 0.25b | A8.25 ± 0.47c | B7.25 ± 0.25b | B7.75 ± 0.25c | B7.75 ± 0.25 b | A8.25 ± 0.25b | C7.00 ± 0.37c | C7.50 ± 0.28c | C7.25 ± 0.25b | B7.25 ± 0.25b |
| T 5 | A8.50 ± 0.28b | A8.25 ± 0.47b | A8.50 ± 0.28a | A9.00 ± 0.05a | C7.00 ± 0.05c | B7.75 ± 0.25c | B7.75 ± 0.25b | B8.25 ± 0.47b | B7.75 ± 0.25a | B7.75 ± 0.25b | C7.25 ± 0.25b | C7.25 ± 0.25b |
| T1: Iron Amino acid chelate; T2: Ferrous sulfate; T3: Ferrous fumarate; T4: Ferric hydroxide poly maltose; T5: Control. Values (Mean ± standard deviation). Values with different small right letters in the same row are significant differed between storage period at p<0.05. Values with different left capital letters in the same column are significant differed between treatments at p<0.05. | ||||||||||||
Table 6: Sensory evaluation of different yoghurt samples during cold storage.
On the other hand, the color was significantly decreased after 3 days in all treatments, except for the first treatment, which color was not significantly affect until seven days of storage. In all treatments, the storage caused a negative impact of flavor, but T1 enhanced yoghurt flavor after 3 days compared to the fresh yoghurt. For texture and overall acceptability, the fresh products in all treatments had highest scores compared to that were stored for 3 days or 7 days. These were in disagreement with those given by Hekmat and McMahon [27] who reported that the consumer panels did not observe significant difference in the appearance of yoghurt fortified with iron. However, all treatments were accepted up to the 7th day of cold storage regarding all taste panel characters evaluated.
In conclusion, iron can be added to yoghurt in different forms without affecting the characteristics of yoghurt with preference the 1st treatment (i.e. yoghurt Fe fortified by amino acid chelate) that showed the best one due to its superior in crude protein, ash, rheological properties and sensory evaluation as well.
Citation: Ziena H, Nasser SA (2019) Iron Fortified Yoghurt: Effect of Different Iron Salts on the Properties of Yoghurt. Adv Dairy Res 7:223.
Received: 25-Feb-2019 Accepted: 12-Apr-2019 Published: 19-Apr-2019 , DOI: 10.35248/2329-888X.19.7.223
Copyright: © 2019 Ziena H, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.