Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2017) Volume 5, Issue 3
Keywords: Chicken pepsin; Proventriculus; Coagulant activity; Vacuum drying; Conservation
The increase in the production and consumption of cheese in the world, on the one hand, and the impossibility of simultaneously increasing the production of rennet on the other, have caused a global supply shortage of this coagulant. These problems are aggravated especially in Muslim countries, for religious reasons, due to the rituals of slaughter. Algeria is no exception. According to the National Office of Statistics (ONS), in 2015, Algerian cheese factories imported approximately 2.25 tons of rennet and / or substitutes, for approximately $ 200,000. According to the same source, nearly 50 000 tons of cheese were sold on the Algerian market, ie a consumption of 1.2 kg / inhabitant / year in 2015 against 0.7 in 2011. Faced to this situation, research on rennet substitutes from different origins were considered.
Pepsin is one of the animal origin proteases that have been experimented for potential use in the cheese industry as substitutes of rennet. In Israel, in 1974, more than 50% of cheese production was prepared from chicken pepsin [1]. Chicken pepsin is known in southern Algeria since a distant era and is used in the manufacture of traditional cheese known as "Takammérit".
In addition, of the pepsin use to curdle milk during the manufacture of cheese, it is also used for a variety of applications in food manufacturing: to make precooked cereals into instant hot cereals, to prepare animal and vegetable protein hydrolysates for use in flavoring foods and beverages It is also used in the leather industry to remove hair and residual tissue from hides [2].
Due to the inadequacy of the bibliography for documents relating to the preservation of enzymes; this work aims to study the stability of chicken pepsin over time. This enzyme is extracted from proventriculus considered as non-recovered waste. This proventriculus salted or not incised or not, were dried under a partial vacuum and then the chicken pepsin residual coagulant activity kinetics during storage was followed.
Berridge substrate
The Berridge substrate is obtained by reconstituting a medium skimmed-milk powder (SOLAREC S.A, Holland) at 12% (w/v) in a solution of CaCl2 (0.01M). To avoid microbial development, 0.04% (w/v) of sodium azide is added. The milk is then stored at 4°C at least 12 hours to ensure the stability and rehydration of the casein micelles.
Chicken pepsin extract
Pepsin extraction was carried out according to Bohak [3] by grinding 100g of chicken forestomachs (proventriculus) in 300 mL of a 30 g NaCl-7g NaHCO3 per litre solution and stirring the mixture for 3 hours at 4°C. After filtering, through gauze (0.5 mm), the crude extract was acidified with 3N HCl to pH 2 for activating the pepsin in 30 min and then readjusted to pH 6.6 with 1N NaOH.
Milk clotting activity
One mL of diluted chicken pepsin solution was added to 10 mL portions of the warmed skim milk (Berridge substrate) for coagulation at 30°C in assay tubes. The coagulation time was determined when the first visible flakes on the moving glass surface appeared. The milk clotting activity was expressed in rennet unit (RU). One RU is the quantity of enzyme contained in 1 cubic centimeter, which can coagulate 10 cubic centimeters of the standard substrate in 100 seconds at 30°C [4]. The RU is functionally expressed as: RU mL-1 = (10 × V)/(T × v), where RU: Rennet unit, V : milk volume, v: enzyme volume, T:milk clotting time (sec).
Proventriculus: recuperation and cleaning
After chicken’s slaughtering and eviscerating, about 700 proventriculus were transported to the laboratory in a cooler. The fat was removed and then washed under a water stream before being drained. A sample is taken for the determination coagulant activity at the fresh state.
Preparation of proventriculus for drying
Proventriculus batches intended for drying under partial vacuum were prepared according to Figure 1. The codes assigned to the different pieces of proventriculus are presented below (Table 1).
| Code | Identification |
|---|---|
| FPOS | Cut in four parts without salt |
| FPWS | Cut in four parts with salt |
| SOS | Incised in slides without salt |
| SWS | Incised in slides with salt |
| WOS | Whole proventriculus without salt |
| WWS | Whole proventriculus with salt |
Table 1: Codes assigned to the different pieces of proventriculus.
The proventriculus salting has been practiced according to the preserving meat traditional method by rubbing them with salt (NaCl) so as to saturate all their surfaces [5]. The proventriculus incision was performed using a scalpel.
Vacuum drying conditions
The prepared proventriculus are distributed on a metal grid (36 cm × 29 cm) separated and lifted about 3 cm from the oven plate. The grid is selected to increase the area of contact between the air and the sample. The drying was carried out in a vacuum oven (GALLENKAMP-SG94/09/405-UK) equipped with three levels of 3132 cm2.
Thus, six batches are placed in the oven, ie two different batches per floor. The drying is monitored periodically until a constant mass is obtained on a control sample for each batch. The drying time is between 48h and 55h. The temperature and the pressure are 47°C ± 3°C and 800 mbar respectively.
Storage of dried proventriculus
After drying under partial vacuum, the dried proventriculus are weighed and packed in aluminum foil and then stored in cardboard boxes at ambient temperature (25 °C) and protected from light and moisture. The storage duration is 54 days.
The experimental data are presented as averages and standard deviations from three experiments.
Residual coagulant activity expression
The residual coagulant activity (333 equivalents Rennet Units) is expressed in terms of relative value to that of fresh proventriculus. It is measured immediately after the drying phase (CAAD) and then during storage (every 9 days up to 54 days storage) (CADS).
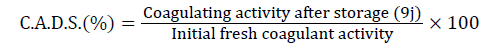
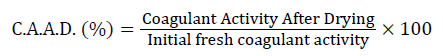
Where: Coagulant Activity After Drying (CAAD),
(coagulant activity during storage) (CADS).
The residual activities of the pepsin solutions extracted from the various parts of the incised or non-incised proventriculus and salted or not subjected to partial vacuum drying are summarized in Table 2.
| Part of the studied proventriculus | Residual activity % (D0) | Residual activity % (D54) |
|---|---|---|
| FPOS | 50 ± 0.6 | 35.5 ± 1.6 |
| FPWS | 22.16 ± 0.8 | 6 ± 1.3 |
| SOS | 45 ± 0.6 | 28 ± 1.2 |
| SWS | 18 ± 0.7 | 4.7 ± 1.1 |
| WOS | 35.5 ± 0.3 | 20.7 ± 1.3 |
| WWS | 25 ± 0.9 | 7 ± 1.6 |
Table 2: Residual coagulant activities of the pepsin solutions extracted from the incised or not incised proventricules and salted or not salted on D0 and D54.
After the drying operation (D0), the residual coagulant activities expressing the yields relative to the fresh condition (333 RU) range from 50% for the four-cut proventriculus without the addition of salt and 18% for proventriculus cut into slides with addition of salt (Table 2, Figures 2 and 3).
At the end of storage, the residual activity was relatively distinct between the proventriculus cut in four parts without salt addition (FPOS): 35.5% and the proventriculus in slides with salt addition (SWS) 4.7% (Table 2, Figures 2 and 3).
To better interpret the results obtained, it is pertinent to study the effect of salting, and of incision and the combined effect of salting and incision on residual coagulant activities of pepsin solutions extracted from vacuum dried proventriculus. In addition, Garcia Fontan et al., [6] report that during the manufacture of dried meat, chemical and physicochemical changes occur, mainly fermentation of carbohydrates and acidification with lipolysis and proteolysis.
These changes could therefore, affect pepsin and consequently its enzyme activity. In addition, salt helps muscle contraction by osmosis and thus promotes the release of water-soluble components to the external environment [7,8]. This could be another factor in the loss of pepsin enzyme activity due to the osmosis and exudation phenomena observed just after salting and created by high salt concentrations.
However, the salt could also have a beneficial effect in the conservation of enzymatic activity by the fact that it could create an inhibitory effect on a wide variety of microbial flora [9]. According to García et al., [10], Vilar et al., [9], and Ravishankar and Juneja [11], the aerobic mesophilic flora may be partially or totally blocked by the halotolerant flora due to the high NaCl concentration on the surface and inside the salted and dried meat pieces. This is the consequence of the water activity (aw) which has undergone a gradual decrease to reach the value of aw 0.755 on the surface and of aw 0.767 inside the dried meat pieces. This factor could contribute to the conservation of pepsin by inhibiting the microbial flora which could degrade it and consequently to deactivate it.
For the incision, its interest was registred in the reduction of the drying duration under partial vacuum thanks to the increase of the contact surface with the air. We note 48 hours of drying for SWS and SOS, 53 hours for FPWS, FPOS and 55 hours for whole proventriculus (WWS and WOS).
Drying is a complex operation involving coupled transfers of material (mainly water) and heat, accompanied by physico-chemical changes and the structure of the material. Heat is transferred by convection of air to the surface; water vapor is removed from the surface of the product by convection.
Drying helps to remove free water by transferring heat which diffuses into the product under the effect of the temperature gradient and consequently to decrease the activity of the water (aw) to a value less than 0.5 which will have the effect of increasing the stability of the dried product during storage [12,13].
The relative stability of the pepsin residual activity was well verified during the storage of the dried proventriculus, with residual activity loss levels depending on the preparation, drying and storage conditions, at the dehydrated state [14].
On the other hand, the combined effect of the salt was noticeably observed during the incision which may have increased enzyme loss by exudation. More proventriculus is incised and salted, greater is the loss of enzymatic activity (Figure 3). The residual activity yields of the whole salted proventriculus are the highest, followed by the salted proventriculus cut in four parts and then the salted proventriculus cut into slides (Figure 3). This differentiation is due to the increase in contact surface area between the proventriculus and the salt (NaCl) which induces exudation and consequently an enzyme loss.
The chicken proventriculus drying helps to reduce the activity of the water and subsequently to preserve in part (up to 50% after drying and 35.5% after drying and then storage) at room temperature for 54 storage days. The incision has a role in reducing the drying duration under partial vacuum (between 48h and 55h); however, it has a negative effect combined with salt in increasing pepsin activity loss. This loss is notable after incision of the proventriculus and is even more accentuated after salt addition. The factors combination study allows us to select the best conditions proposed:
The pepsin residual coagulant activity from proventriculus cut into four parts, unsalted and dried under partial vacuum for 53 h at 47°C., expressed satisfactory yield after drying of 50% with better stability during storage period of 54 days (35.5%) compared to other factor combinations used for dried proventriculus preparations.