Advances in dairy Research
Open Access
ISSN: 2329-888X
ISSN: 2329-888X
Research Article - (2019)Volume 7, Issue 2
Objective: To determine whether the addition of polyphenols (gallic and caffeic acids) to infants milk substitutes, prevents oxidation of unsaturated fatty acids, under conditions of oxidative stress.
Materials and methods: In order to induce controlled oxidative stress we used ultraviolet radiation UV-C 254 nm with energy doses in the range of 0-14 J/ml. We followed lipid peroxidation by the thiobarbituric acid reactive substances (TBARS) assay in order to measure the amount of secondary oxidation products, malondialdehyde (MDA). Polyphenols were added to the type specified the range of 0-3 mM concentrations. To measure the change polyphenols amount, the method of Folin-Ciocalteu assay was used.
Results: We have proved that UV-C radiation is able to oxidize PUFA. We found a direct relationship between radiation dose and the amount of oxidation products formed as a result of exposure to radiation. We showed that the polyphenols, gallic acid and acid coffee, are able to decrease oxidation by the observed decline in oxidation products of MDA. High concentrations of polyphenols can prevent oxidative damage radiation exposure: coffee acids are effective in about 20% more than gallic acid.
Conclusion: We recommend adding acid coffee to newborns milk powder in order to protect PUFA fatty acids, as well as to allow additional nutritional benefit to the newborn.
Lipid peroxidation; Infant formulas; Ionizing radiation; Polyphenols
Unsaturated fatty acids play an important role in various physiological, biochemistry, nutrition, medicine, and food. Unsaturated fatty acids are also a target for oxidative damage. Lipid peroxidation is a complicated process that leads to the formation of many compounds [1,2]. Lipid peroxidation products seem to be directly involved in the development of cancer, atherosclerosis, and aging processes [3]. Malondialdehyde (MDA), in fact known to be a mutagenic species, is one of the end products of lipid peroxidation [4,5].
The oxidative degradation of polyunsaturated fatty acids contributes significantly to the reduced shelf life of many products [6]. Some of its effects include the development of off-flavors and odors, changes in texture and loss of nutritive value [7]. The long chain polyunsaturated fatty acids (LCPUFA), docosahexaenoic acid C22:6 ω-3 and arachidonic acid C20:4 ω-6 found to be important in the development of brain and retina in utero [8]. The European Society for Pediatric Gastroenterology recommended that formulas for preterm infants should be supplemented with LCPUFA, and various formulations are now commercially available [9]. However, the European Society for Pediatric Gastroenterology and Nutrition stressed that increasing polyunsaturated fatty acid content in infant formula may predispose babies to oxidative damage. The susceptibility of lipids to oxidation is a major cause of quality deterioration in food emulsions [10].
Polyphenols are compounds containing at least one structure or more of the aromatic benzene ring, to which hydroxyl group is attached to the side (one or more). In recent decades, polyphenols were investigated because of their health benefits. Polyphenols found to exert a protective action on human health and are key components of a healthy and balanced diet [11,12]. Long term consumption of diets rich in plant polyphenols offer protection against development of cancers, cardiovascular diseases, diabetes, osteoporosis and neurodegenerative diseases [11,12]. The ability of the polyphenols to interfere with the oxidation activity and serve as an antioxidants is due to their ability to donate a hydrogen atom. They react with peroxide radical generated in the initial stage, producing stable hydro-peroxides parallel number of structural resonance in the molecule [12].
Infant formula is an emulsion that contains unsaturated fatty acids that are very sensitive to oxidative stress. Infant formulas should be aimed at providing the best alternative to breast milk for the babies of women who are unable to continue breastfeeding until 6 months of age, and it should form an adequate substitute for human milk, approaching the structural and functional effects observed in breastfed infants after they reach 6 months of age. In order to study whether polyphenols such as caffeic and gallic acids have protective effect against oxidative damage, we used infant formula, containing unsaturated fatty acids, as an in vitro model of a biological system.
The aim of this study was to determine whether polyphenols such as gallic acid and caffeic acid can protect baby dairy-based milk from damage caused by direct exposure to controlled oxidative stress. We were looking to improve the shelf span and resistance of dairy formula while adding a nutritional benefit to the infant. We simulate an oxidative stress conditions that appears naturally in the body.
All solutions were prepared from analytical-grade chemicals and distilled water that was passed through a Millipore setup at a final resistance that was above 10 MΩ/cm.
All reagents and solvents were of analytical grade. Trichloroacetic acid (TCA), thiobarbituric acid (TBA), and butylated hydroxyl toluene (BHT), gallic acid (GA) caffeic acid (CA), ethyl alcohol, sodium carbonate and Folin–Ciocalteu solution were purchased from Sigma-Aldrich. Infant milk formula was purchased at the local market.
Milk samples
One of the commonly used newborn dairy formula stages 1 (DF) was studied immediately after opening the milk powder container (The brand name "Materna" is an Israeli commercial formula). The lipid and antioxidant compositions of the milk powderare listed in Table 1 (according to the manufacturers report on the package). 20:4(ω-6), Arachidonic acid (ARA), and 22:6(ω-3) docosahexaenoic acid (DHA) are added to milk powder by the manufacturer. The milk suspension was prepared by adding 10.0 g of powder to 100 ml of distilled water and then mixing well.
| Ingredients in 100 g powder of dairy milk formula | |
|---|---|
| Total fats (g) | 26.5 |
| Saturated fats (g) | 11.8 |
| Unsaturated fats (g) | 14.7 |
| Linoleic acid (mg) | 3600 |
| Linolic acid (mg) | 380 |
| ARA (mg) | 100 |
| DHA (mg) | 100 |
| Vitamin E (mg) | 10 |
| Vitamin C (mg) | 90 |
| Vitamin A (IU) | 1500 |
| Iron (mg) | 5.9 |
Table 1: Formula composition.
Induction of oxidative damage
5 ml of milk were transferred to a plastic Petri dish with a diameter of 5.0 cm. Plate was placed in a UV-R device within 5 cm from the lamp at room temperature for various time periods, which were determined in advance [13]. Then the solution was collected from the flask into a glass container and was staying for 30 min, before assay.
Determination of total polyphenols content
The total phenolic content in the seminal fluid was determined by using the Folin–Ciocalteu method [14]. This method is based on the redox reaction of the reagent, forming a blue color pigment, with typical absorbance at 760 nm. All UV-Vis measurements were performed using a Varian Cary Bio 100 UV-vis spectrophotometer. 1.0 ml of the milk was transferred to a tube, following by adding 2.0 ml of extracting mixture composed of 80% ethanol and 20% water by volume in order to extract the polyphenols. The mixture was vortexed for 10 seconds, and then was centrifuged for 15 minutes for sedimentation of solids at 5,300 rpm. An aliquot of 1.0 ml from the supernatant was mixed with 1.0 ml of Folin reagent and 2.0 ml saturated solution of sodium carbonate for assay. The solution was vortexed for 15 seconds, and kept in dark for 30 minutes for color development. The solution was centrifuged (5,300 rpm) for 10 minutes until it was transparent. The supernatant was collected and the solution absorbance was measured by using UVVis spectrophotometer at wavelength of 760 nm against blank. The total amount of polyphenols was expressed as Gallic acid equivalents (GAE), based on the calibration curve (R=0.99).
Lipid peroxidation measuring
We follow the lipid peroxidation by the thiobarbituric acid reactive substances (TBARS) assay in order to measure the amount of secondary oxidation products, malondialdehyde (MDA) levels were measured spectroscopically according to a procedure already published by Fenaille et al., with minor modifications [15]. The assay based on the thiobarbitoric acid (TBA) reaction; a reaction between oxidized lipids and solution of 2- thiobarbitoric acid under acidic conditions to yield a pink chromogen with a maximum absorbance at 532 nm [15].
An aliquot of the milk (1 ml) was transferred to a 5 ml tube, followed by successive additions of TBA 1% in TCA 5% and BHT 0.8% in ethanol. The mixture was then homogenized and centrifuged at 5,300 rpm for 10 min and incubated in a 70°C water bath for 20 min. Samples were subsequently cooled under tap water for 5 min and centrifuged for 5 min to separate flocculent material. The color produced by the chemical reaction was read at 532 nm against a blank reaction mixture and the amount of MDA formed was determined by using the molar extinction coefficient 532 nm)=1.56 × 10-5 cm nmol-1 [16].
Spectrophotometric measurements
All U.V.-Vis measurements were performed using a Cary 100 Bio, UV-Visible spectrophotometer.
Production of radicals
The ionizing radicals were formed by irradiating the suspension with ultraviolet lamp (UV-c) 254-365 nm (The UV device contains a dark compartment) Model CN-6 made by VILBER-LOURMAT (230 V, 50-60 Hz). When ionizing radiation is absorbed by a dilute aqueous solution, Hydroxyl Radicals (OH) and superoxide radicals (O2-) are formed with high utilization [17]. In the presence of oxygen in the solution there is a production of singlet oxygen; 1O2 [18]. Likely as milk contains unsaturated fatty acids, UV radiation can directly attack the double bonds, and make direct oxidation damage to the unsaturated fatty acids. All statistics were performed using the SPSS 11.0 program for Windows. Values of variables were expressed as mean ± SD.
Radiation doses of 0-3 J/ml caused almost no oxidative damage to the fatty acid in milk substitutes, and MDA levels were maintained around 12.0 ± 1.7 nmol/g.
In order to determine the effect of polyphenols on the level of oxidative damage caused to the fatty acids constant radiation dose of 9.54 J/ml was chosen. Milk samples have been exposed to radiation intensity of 9.54 J/ml, in the presence of various concentrations of polyphenols; Gallic acid (GA) and Caffeic acid (CA). Results are shown in Figure 1. Percentage of preventing oxidative damage by gallic acid and caffeic acid are summarized in Table 2.
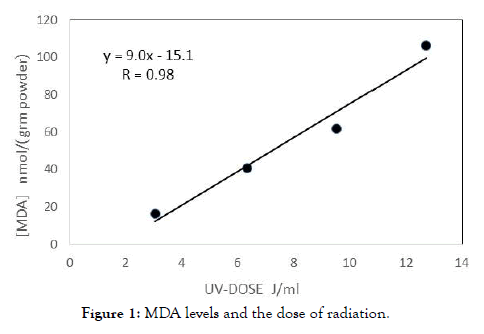
Figure 1. MDA levels and the dose of radiation.
| Polyphenols types | UV-C DOS | Polyphenols concentration | % Protection from oxidation |
|---|---|---|---|
| Gallic acid | 9.54 J/ml | 1 mM | 10 ± 2% |
| Gallic acid | 9.54 J/ml | 2.5 mM | 11 ± 5% |
| Caffeic acid | 9.54 J/ml | 1 mM | 12 ± 2% |
| Caffeic acid | 9.54 J/ml | 2.5 mM | 13 ± 3% |
Table 2: Oxidative damage prevention by gallic acid and caffeic acid.
As shown in Table 2 when the concentration of both gallic acid and caffeic acid were increased from 1 mM to 2.5 mM, preventing oxidation percentage has increased more than 40%. Caffeic acid protect from oxidation about 25% more than gallic acid.
In order to prove that the addition of polyphenols to the milk formula was the reason for the decline in oxidative damage that was caused to the fatty acids, we carried out an experiment that examines the amount of polyphenols in the system before irradiation and after irradiation in two doses of UV-C, and for two effective concentrations of polyphenols. Results for the influence of Gallic acid and Caffeic acid in 1.0 mM concentration are shown in Figure 2.
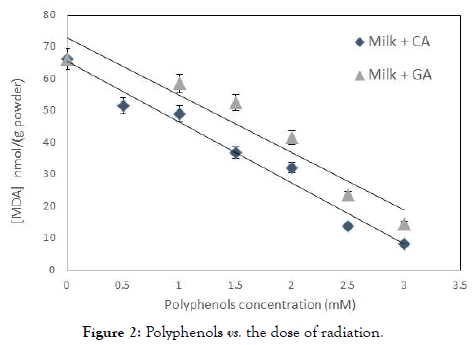
Figure 2. Polyphenols vs. the dose of radiation.
We believe that the reason for this is the high initial levels of antioxidants which are added to milk substitutes. We measured an initial amount of 10.0 ± 0.9 mg GA/g of polyphenols in the milk substitutes, immediately after opening the box. Linear dependence between MDA levels and the dose of radiation received from 3.0 to 13 J/ml (Figure 3). The aim of this experiment was to find conditions in which cause oxidative damage to the fatty acids in the milk substitutes; significant and linear damage. This is to test whether a proactively addition of polyphenols, reduce the damage.
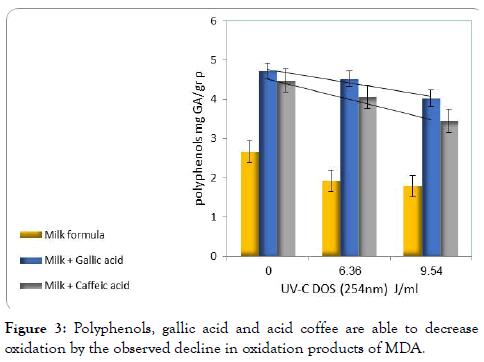
Figure 3. Polyphenols, gallic acid and acid coffee are able to decrease oxidation by the observed decline in oxidation products of MDA.
Caffeic acid and gallic acid decreased oxidative damage of fatty acids in correlation to the concentration of polyphenols. Caffeic acid has systematically beneficial effect compared with gallic acid. Addition of 3 mM polyphenols reduced the amount of MDA to the initial amount of 11 nmol/g. Therefore, under these conditions, it is possible to completely neutralize the oxidative damage caused to the fatty acids, in baby milk formula, in exposure to radiation dose of 9.54 J/ ml.
It is evident that milk substitutes have an initial quantity of polyphenols. This is likely due to the fact that cow's milk is the source for the preparation of baby formula, and cow's milk naturally contains polyphenols. However, it seems they did not protect the powder from oxidative damage. It is likely that the reason for the unprotect ability is found in the type of polyphenols low activity, and its low concentration that may be below the critical protection threshold.
When drawing the amount of polyphenols, versus the dose of radiation, there is a linear decrease in the concentration of polyphenols. As the initial concentration of polyphenols was higher, the slope of the graph was greater. Namely, more polyphenols consumed for each single photon of radiation, and less MDA production. Caffeic acid in concentration of 0.5 mM responded to seven molecules of polyphenols, for every 100 photons, a concentration of 1.0 M responded about 10 molecules of polyphenols per 100 photons, whereas concentrations of 2.5 mM, responded to about 30 molecules of caffeic acid per 100 photons. The efficiency of gallic acid was lower by about 15% than that of caffeic acid.
Simple calculation shows that in 1.0 L of milk, there are approximately 50 mM of unsaturated fatty acids (and an average of 150 mmol of double bonds). Both polyphenols, caffeic acid and gallic acid, at a concentration of 3.0 mM were able to reduce the damage of the fatty acids to the initial values (baseline). This is probably because in the emulsion, the fatty acids are organized in micelles, and most of them are not in contact with radicals generated during radiation. Only the exposed portion to water is oxidized. This fraction is protected by the polyphenols in the aqueous solution.
In this study we found that polyphenols such as gallic acid and caffeic acid have an important role in preventing oxidative damage caused to infant formula by ionizing radiation. Caffeic acid was slightly more effective than gallic acid, however the concentration factor affects more than the polyphenol itself; the higher the concentration of polyphenols, the better the protection.
Given the results of this work, we recommend adding caffeic acid to the powdered infants' milk substitutes. This addition will protect the fatty acids particularly the important LC-PUFA from environmental oxidative damage. In addition, it is also important in protecting the formula from oxidative stress and damage which may have devastated result on the baby consumer. The major advantage in adding polyphenols in addition to lycopene or vitamin C is a high resistance of caffeic acid over time, and its heating resistance.
Citation: Saphier O, Barak T, Hamou B, Silberstein T (2019) Caffeic and Gallic Acids Protect Commercial Infant Milk Formula from Oxidative Damage. Adv Dairy Res 7:224.
Received: 30-Mar-2019 Accepted: 22-Apr-2019 Published: 30-Apr-2019 , DOI: 10.35248/2329-888X.19.7.224
Copyright: © 2019 Saphier O, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.