Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2014) Volume 5, Issue 3
In this review article the basic principle of separation process during Micellar electro kinetic chromatography (MEKC) are described. The separation mechanism in MEKC is based on differences in equilibrium between an aqueous Phase and micellar Phase. These Phases are moving different velocities, due to a combination of electrophoresis & electro osmosis. It is a useful branch of capillary electrophoresis (CE) that utilizes surfactant above critical micelle concentration (CMC) as pseudo-stationary phase. MEKC can be employed to separate both charged and neutral molecules, individually or simultaneously, including chiral compounds. MEKC benefits from high peak efficiency due to electro-osmotic flow (EOF) in the separation capillary, compounded with large variety of synthetic surfactants, organic modifiers, temperature and variable separation voltage has made MEKC the method of choice for separation scientists. In this review, we present the introduction of CE, fundamentals of surfactant chemistry as it relates to MEKC, separation principles in MEKC including equations involved in calculating separation parameters (capacity factor, resolution etc.).
Keywords: MEKC; Electrophoresis; Electro osmosis; Electro osmotic flow; Capacity factor
According to International Union of Pure and Applied chemistry (IUPAC), chromatography can be defined as: “A Physical method of separation in which the components to be separated are distributed between 2 phases, one of which is stationary (stationary phase) while the other (the mobile phase) moves in a definite direction”. The mobile phase flow can be controlled by gravity (e.g., column chromatography), by applying pressure (e.g., high pressure liquid chromatography), and by electricity (e.g., electrophoresis).Capillary Electrophoresis (CE) is an electro-driven separation techniques, it calls for low reagent consumption, high efficiency and selectivity with reasonably short analysis time. In CE, the capillary is filled with suitable buffer and after injecting analytes from anode side (under normal polarity conditions), high voltage is applied at its both ends. The analytes (positively and negatively charged) will move with different velocity and can be separated based on their electrophoretic mobility. However, in case of neutral molecules, since they do not bear any charge, move with the solvent front and elute as a single band and thus, cannot be separated. To solve this problem, charged surfactants above Critical Micelle Concentration (CMC) are added in the CE running buffer, which allows separation of uncharged molecules along with charged ones. Electrokinetic chromatography (EKC) is a family of electrophoresis techniques named after electrokinetic phenomena, which include electro osmosis, electrophoresis, and chromatography. Miceller electrokinetic chromatography (MEKC) is a mode of EKC in which surfactants (micelles) are added to the buffer solution. Surfactants are molecules which exhibit both hydrophobic and hydrophilic character. They have polar “head” groups that can be cationic, anionic, neutral, or zwitterion and they have nonpolar, hydrocarbon tails. The formation of micelles or “micellization” is a direct consequence of the “hydrophobic effect.” The surfactant molecules can self-aggregate if the surfactant concentration exceeds a certain critical micelle concentration (CMC). The hydrocarbontails will then be oriented toward the center of the aggregated molecules, whereas the polar head groups point outward. Micellar solutions may solubilize hydrophobic compounds which otherwise would be insoluble in water. The front cover picture shows an aggregated SDS molecule. In the center of the aggregate, p-fluorotoluene is situated depicting the partitioning of a neutral, hydrophobic solute into the micelle. Every surfactant has a characteristic CMC and aggregation number, i.e., the number of surfactant molecules making up a micelle (typically in the range of 50-100). The size of the micelles is in the range of 3 to 6 nm in diameter; therefore, micellar solutions exhibit properties of homogeneous solutions. Micellar solutions have been employed in a variety of separation and spectroscopic techniques. In 1980, Armstrong and Henry pioneered the use of micellar solutions as mobile phase’s forreversed-phased liquid chromatography (RPLC).
MEKC is based on the addition to the buffer solution of a micellar “pseudostationary” phase, which interacts with the analytes according to partitioning mechanisms, just like in a chromatographic method. The “pseudostationary” phase is composed of a surfactant added to the buffer solution in a concentration above its critical micellar concentration (CMC). In this system, EOF acts like a chromatographic “mobile phase”. From a “chromatographic point of view”, the EOF’s “plug-like” flow profile is almost ideal as it minimizes band broadening, which can occur during the separation process. The most commonly used surfactant sodium dodecyl sulfate (SDS), an anionic surfactant. The anionic SDS micelles are electrostatically attracted towards the anode. The EOF transports the bulk solution towards the negative electrode due to the negative charge on the internal surface of the silica capillaries. But the EOF is usually stronger than the electrophoretic migration of the micelles and therefore the micelles will migrate also toward the negative electrode with a retarded velocity (Figure 1).
When a neutral analyte is injected into the micellar solution, a fraction is incorporated into the micelle, while the remaining fraction of the analyte migrates with the electro-osmotic velocity. Consequently, micelles decrease selectively the migration of neutral solutes they interact with (by partitioning mechanism), which otherwise would migrate with the same velocity as the EOF.
The separation depends on the individual partitioning equilibrium of the different analytes between the micellar and the aqueous phase. The greater percentage of analyte is distributed into the micelle, the slower it will migrate. Therefore, analytes that have greater affinity for the micelles exhibit slower migration velocities compared with analytes that are mostly distributed in the bulk solution with SDS micelles, the general migration order will be exactly the opposite as in ECZ: anions, neutral analytes and cations. Anions will remain mostly in the bulk solution due to electrostatic repulsions from the micelle; neutral molecules will be separated exclusively due to their hydrophobicity; while cations will migrate last due to the strong electrostatic attraction [1,2]. This generalization regarding the migration order can be sometimes useful, but strong hydrophobic interaction between analytes and micelles can overcome repulsions and attractions. Likewise, the own electrophoretic mobilities of the analytes can also modify the migration order. Analytes which are highly retained by the micelle will have longer migration times, close to the EOF (t0). Very hydrophobic compounds may be totally included into the micelle and will migrate with the micelles velocity (tmc). Methanol is not retained by the micelles and migrates with t0 being used as marker for the EOF, while a dye Sudan III is totally included into the micelle and can be used as a micellar marker. The period between the migration time of the bulk solution and the migration time of the micelle is often referred to in the literature as migration time window (Figure 2).
A relatively recent development in MEKC has been to perform separations in the absence of EOF. This may be achieved using coated capillaries or at low pH values. This could be especially useful in the separation of acidic analytes, which would ionize at high pH values and would not interact with the negatively charged SDS micelle. Cationic surfactants can be used in MEKC to reverse the charge on the capillary wall, by absorption on the capillary wall surface through a mechanism involving electrostatic attraction between the positively charged ammonium moieties and the negatively charged Si-O-groups; when a reversal of the EOF takes place.
Ionic surfactants are essential for MEKC. Numerous ionic surfactants are commercially available. The surfactants suitable for MEKC should meet the following criteria
1. The surfactants must have enough solubility in the buffer solution to form micelles.
2. The micellar solution must be homogeneous and UV transparent.
3. The micellar solution must have a low viscosity.
Table 1 lists the CMC, aggregation number, and Kraft point of some selected ionic surfactants available for MEKC. The Kraft point is the temperature above which the solubility of the surfactant increases steeply due to the formation of micelles. In order to obtain a micellar solution, the concentration of the surfactant must be higher than its CMC. The surfactants enough solubility to form micelles only at temperatures above the Kraft point as mentioned above. The counter ion of the ionic surfactant doesaffect the Kraft point. For example, the Kraft point of sodium dodecyl sulfate (SDS) is 16°C but potassium dodecyl sulfate has a Kraft point of approximately 35°C. Therefore, if SDS is dissolved in a buffer containing potassium ions, the solubility of SDS will be less than its CMC at ambient temperature because of the exchange reaction of the counter ions. The actual CMC in the buffer solution is usually lower than the value listed in Table 1, as these values were obtained with pure water as solvent. High concentrations of surfactant (>200 mM) result in relatively high viscosities (and high currents) and should therefore be avoided in MEKC. It is recommended that the concentration of the buffer salt should not be lower than 10 mM. A higher concentration of buffer relative to that of surfactant is preferred to keep the pH constant during the run. It should be remembered that, in electrophoresis, electrolysis occurs at the electrodes, resulting in a reduction of the pH of the anodic solution. This solution may enter the capillary during the run when an anionic micelle (e.g., SDS) is employed. The cathodic solution becomes alkaline during electrophoresis and may enter the capillary when a cationic surfactant (e.g., CTAB) is employed. In this case the EOF is reversed.
| Name of Surfactant | CMCa/10-3 M | n | Kp |
|---|---|---|---|
| Sodium dodecyl sulfate (SDS) | 8.1 | 6.5 | 16 |
| Sodium tetradecyl sulfate (STS) | 2.1(50°C) | 138b | 32 |
| Sodium decanesulfonate | 40 | 40 | - |
| Sodium dodecanesulfate | 7.2 | 54 | 37.5 |
| Sodium N-lauroyl-N-methyltaurate (LMT) | 8.7 | - | <0 |
| Sodium polyoxyethylene dodecyl ether sulfate | 2.8 | 66 | - |
| Sodium N-dodecanoyl-L-valinate (SDVal) | 5.7(40°C) | - | - |
| Sodium cholate | 13-15 | 2-4 | - |
| Sodium deoxycholate | 4-6 | 4-10 | - |
| Sodium taurocholate | 10-15 | 5 | - |
| Sodium taurodeoxycholate | 2-6 | - | - |
| Potassium perfluoroheptanoate | 28 | - | 25.6 |
| Dodecyltrimethylammonium chloride (DTAC) | 16(30°C) | - | - |
| Dodecyltrimethylammonium bromide (DTAB) | 15 | 56 | - |
| Tetradecyltrimethylammoniumbromide (TTAB) | 3.5 | 75 | - |
| Cetyltrimethylammonium bromide (CTAB) | 0.92 | 61 | - |
| a25°C bIn 0.10M NaCl |
|||
Table 1: Critical Micelle Concentration, Aggregation Number (n), and Kraft point (Kp) of Selected Ionic Surfactants.
The surfactants are amphiphilic in nature and are miscible with both polar and apolar substances. A typical amphiphilic molecule itself consists of polar (hydrophilic) group (e.g., alcohol, ether, carboxylate, sulfate, sulfonate, phosphate, amine, ammonium etc) and apolar (hydrophobic) group (e.g., usually a long hydrocarbon chain) as represented in Figure 3.
The hydrophilic portion exhibits a strong affinity for water, while the hydrophobic part tends to accumulate together (hydrophobic effect) due to mutual antipathy for water. Because amphiphiles conation both water loving and repelling groups, they often tend to migrate at the interface of an aqueous solution, such that hydrophilic part is in water and hydrophobic part away from water (in the air) as represented in Figure 4.
Due to accumulation at the air?water interface, the surface tension of water drops and these molecules are accordingly dubbed, surface active agents. There are many substances, such as medium? or long?chain alcohols that are surface active (e.g., n?hexanol, dodecanol) but they are not considered as surface?active amphiphiles (surfactants). Specifically, surfactants are distinguished by self?assembly structures (micelles, vesicles) in bulk phases [3-9] and ability to form oriented monolayers at the interface. Surfactants are also responsible for the fundamental physical effects, such as, wetting, dispersion or deflocculation and emulsification. Alternatively, surfactants interfere with the ability of the molecules of a substance to interact with one another (especially at the interface) and thereby, lower the surface tension of the substance.
Surfactants are characterized based on the charge present in the hydrophilic portion of the molecule (after dissociation in aqueous solution). There are four categories of surfactant
(1) Anionic, (2) Cationic, (3) Nonionic and (4) Zwitterionic.
Anionic surfactants, when dissolve in water, dissociate into hydrocarbon chain bearing anion (e.g., ?COO-, ?SO3-, ?PO4-3, ?SO4-2), and a counter cation (e.g., Na+, K+) and are the most commonly used type of surfactants. Cationic surfactants on the other hand, when dissolved in water, dissociate into hydrocarbon chain bearing cationic head group [e.g., (R)4N+, (R)4P+] and a counter anion (e.g., Cl-, Br-). A very large proportion of this class corresponds to fatty amine salts and quaternary ammoniums, with one or several long chain of the alkyl type, often coming from natural fatty acids. The quaternary ammonium group containing surfactants are well known for displaying emulsifying properties, antimicrobial activity, anti-corrosive effects and are used in cosmetic formulations and as phase transfer catalyst in organic synthesis. Zwitterionic surfactants contain both anionic and cationic portion within the surfactant backbone and are also known as amphoteric surfactants. Some zwitterionic surfactants stay zwitterionic at all pH, while few are cationic at low pH and anionic at high pH. They are generally quite expensive as they are not very easy to make and thus are used in special circumstances, for instance in cosmetics, due to high biological compatibility and low toxicity. Nonionic surfactants, as name indicates, are devoid of charges. The hydrophilic group usually is alcohol, phenol, ether, ester or amide. Large proportions of these nonionic surfactants are hydrophilic by the presence of a polyethylene glycol chain and are called polyethoxylatednonionics. Sugar?derived nonionic surfactants are also in use as they exhibit very low toxicity and good have excellent biodegradability.
Since MEKC is often applied in the separation of analytes with very similar hydrophobicities and chemical characteristics, sometimes is useful to extend the concept of using a “mobile phase” and a “pseudostationary phase” to the use of buffer additives such as organic modifiers and cyclodextrines. Organic solvents (methanol, acetonitrile) are used in CZE in order to increase solubility of the analytes, but their role in MEKC is more complex and profound. Organic solvents reduce EOF, consequently increase the migration times and migration time window of the analytes. Also, organic additives reduce the hydrophobic interactions between the micelle and the analyte and can be useful in the separation of analytes which otherwise are almost completely incorporated in micelles. The addition of organic solvents will increase the migration velocity of these hydrophobic analytes, by reducing the partition coefficient between the micelle and the bulk solution. However high concentration of organic solvents may break down the micellar structure, consequently concentrations above 25-30% should be avoided. Cyclodextrines (CD) are cyclic oligosaccharides with truncated cylindrical molecular shapes, having an external hydrophilic surface and an internal hydrophobic cavity, in which they can include other compounds by hydrophobic interactions. The inclusion mechanism is sterically selective, because analytes must fit the size of the cavity, the diameter of which depends on the number of glucose units in the CD structure. There is a wide range of both natural and derivatised CD commercially available. The native CD, α-, β-, and γ-CD possess different numbers of glucose sub-units, six, seven and eight respectively. These surface hydroxyl groups can be chemically replaced with groups such as hydroxypropy and dimethyl groups. Ionic chargeable CD offers the possibility of separation of neutral drug enantiomers or enhanced separation of ionic drugs. Several CE specific derivatised CDs have been produced with amino, sulfate or carboxylic groups. Because of the chirality of the hydroxyls in the glucose molecules that form the rim of the CD cavity, the inclusion complex formation will be chirally selective. If the enantiomers of a compound have different binding constants, then chiral separation is possible by adding the proper CD in the buffer electrolyte. CDs are neutral from electrophoretic point of view, and are not incorporated in micelles, because of the hydrophilic nature of the outside surface of the molecules. Therefore, an analyte included in the CD will migrate with the same velocity as the EOF. The addition of cyclodextrines reduces the apparent distribution coefficient of the analytes between the two phases. Hydrophobic analytes can become incorporated into either the CD cavity or the micelle. Effectively the addition of the CD establishes two “pseudo stationary” phases in the electrolyte, which can reduce analysis times and offer the possibility of improved separation. CDs have advantages over organic solvents, as they are UV transparent and non-volatile. The schematic principle in cyclodextrin modified micellarelectrokinetic chromatography is presented (CD-MEKC) (Figure 5).
In order to calculate the capacity factor, it is necessary to know the migration time of the bulk solution, t0, the migration time of the micelle, tmc, as well as the migration time of the analyte, tR. Since the whole capillary is filled with micellar solution, the markers of the bulk solution and the micelle are required to obtain t0 and tmc. Strictly speaking, no ideal marker is available for MEKC. The marker for the bulk solution must be electrically neutral as well as totally excluded from the micelle. Mesityl oxide, often used in CZE to measure t0, is not an appropriate choice in MEKC, because it is partially incorporated into the micelle. Methanol often serves to measure t0, because its distribution coefficient is almost negligible. Furthermore, it can be detected by UV absorption due to a change in refractive index as the methanol peak passes through the detection zone. The marker for the micelle must be totally incorporated into the micelle. Sudan III or IV are often used to measure tmc. Both solutes are not soluble in water and can be dissolved in methanol or in the micellar solution. However, because of the poor solubility in water, it is not always possible to observe the peaks of Sudan III or IV in the electropherograms. As an alternative, compounds that are insoluble in water but soluble in the micellar solution can be employed to measure tmc. Timepidium bromide or quinine hydrochloride are good markers for anionic SDS micellar systems [10,11].
In MECC we can define the capacity factor (k) similarly as in chromatography
k = nmc/naq
where nmc and naq are the amount of analyte incorporated into the micelle and in the aqueous respectively. It can be calculated from the migration time of the analyte (tR), of the EOF (t0) and of the micelle (tmc)
k = tR-t0/t0(1-tR/tmc)
When k = 0, the migration time of the analyte is equal to t0, which means that the analyte does not interact with the micelle; and when k is infinity, the migration time of the analyte is equal to tmc, which means that the analyte is totally incorporated into the micelle.
The capacity factor is a fundamental term in chromatography while the electrophoretic mobility is characteristic to the electrophoretic process. In ECZ the migration velocity (v) of the analyte is expressed as
v = (μeo + μa) E
Where μeo and μa are the electrophoretic mobilities of the EOF and analyte respectively and E is the electric field strength. We can apply this equation to MECC by defining the effective electrophoretic mobility of a neutral analyte (μna) as
μna = μmc k/1+k
Where μmc is the electrophoretic mobility of the micelle and k/1+k represents the fraction of analyte incorporated into the micelle. Thus, the velocity of a neutral analyte in MECC is given as
v = (μeo + μna) E
The capacity factor provides quantitative information about the analyte distribution between the two phases, while the electrophoretic mobility only gives qualitative information about it.
The resolution equation in MECC can be given by the following equation
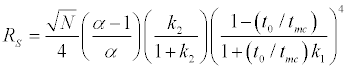
Where N is the theoretical plate number, α the separation factor between the two analytes and k1 and k2 their capacity factor. The separation factor (α) is determined by the micellar solubilization process and is influenced by the chemical nature of both the micellar phase and the surrounding aqueous phase. Various surfactant systems can be used as well as mixed micelles, possessing different solubilization characteristics, in order to control migration behavior of the analytes and optimize selectivity.
In the pharmaceutical industry, the determination of the optical purity or separation and determination of enantiomers is becoming increasingly important. The non-active enantiomer in a drug formulation may be considered as an impurity. High resolution separation methods are required to achieve chiral separations. The approaches used in CE, discussed next, are relatively simple compared to HPLC methods in which expensive, chiral stationary phases are frequently used. In CE only minute amounts of chiral selectors are required to determine enantiomeric purity. Two approaches can be used to perform enantiomeric separations in MEKC
1. Use of chiral surfactants
2. Use of chiral additives
Chiral surfactants
Bile salts are widely available commercially and have shown to be useful chiral surfactants. Sodium cholate or sodium deoxycholate can be used under neutral or alkaline conditions to ionize the carboxyl group of the surfactant. Taurine conjugates of bile salts can also are used in acidic conditions because taurine has a sulfonic acid group. Amino acid-derived surfactants (e.g., sodium N-dodecanoyl-Lvalinate (SDVal) are another group of chiral surfactants that are commercially available. They also must be used under neutral or alkaline conditions. In order to use these surfactants under acidic conditions, SDS can be added to form mixed micelles with appreciable electrophoretic mobilities. The addition of a small amount of methanol and/or a relatively high concentration of urea often improves resolution while sharpening peak profiles.
Chiral Additives
The second, more popular, method of enantiomeric separation by MEKC is to add CD to the micellar solution. The SDS micelle may be conveniently used for this approach. Various CDs or CD derivatives may be tried. The concentrations of SDS and CD should be optimized to yield optimal capacity factors. Cicletanine is a member of a new class of antihypertensive drugs, the fusopyridines. Chiral selectivity was obtained by using a buffer consisting of 100 mM borate, 50 mM SDS, pH 8.6 and 50 mM b-CD. The addition of methanol and/or urea often enhances solubility and improves resolution. When g-CD is employed, a second chiral component, such as d-camphor-10-sulfonate or l-menthoxyacetic acid, may enhance resolution.
Photometric detection (LOD is over 10-3 M) (Figure 6).
Laser induced fluorescence (LIF) detection (LOD is below10-9 M) (Figure 7).
Electrochemical Detection (Figure 8).
In principle MEKC is used for the analysis of neutral compounds, or when analyzing mixtures of neutral and charged solutes. But MEKC conditions are also employed when selectivity requirements for a separation exceed the simple mobility differences obtainable in CZE.
MEKC can be especially useful for the determination of drugs in samples having a high protein content (clinical samples, biofluids) reducing the disadvantageous matrix effects caused by organic materials, while CZE through its simplicity and operation stability could be advantageous for pharmaceutical determinations.
MEKC can be usually applied in simultaneous separation from complex mixtures of pharmaceutical substances with very similar structural and physico-chemical characteristics. Many reports have been published detailing the use of MEKC for pharmaceutical applications; Table 2 presents briefly selected pharmaceutical applications and the description of the electrophoretic conditions.
| Pharmaceutical class | Substances | Electrophoretic conditions |
|---|---|---|
| Penicillins | Amoxicillin, Ampicillin, Benzylpenicillin, Phenoxymethypenicillin, Oxacillin, Cloxacilin | 40 mM sodium tetraborate + 100 mM SDS, pH – 9.3 voltage: + 10kV, temperature: 20°C, UV detection 210 nm |
| Cephalosporins | Cefazoline, Cefuroxime, Ceftriaxone, Cefoperazone, Ceftazidime | 20 mM sodium tetraborate + 15 mM disodium hydrogenophosphate + 50 mM SDS pH – 6.5 voltage: + 18kV, temperature: 20°C, UV detection 214 nm |
| Macrolides | Erythromycin, Tylosin and related substances | 80 mM sodium phosphate + 20 mMsodium cholate + 7 mMAcetyltrimethyl ammonium bromide, pH – 7.5 voltage: + 15kV, temperature: 25°C, UV detection 280 nm |
| Aminoglycosides | Gentamicin, Sisomicin, Netilmicin, Kanamycin, Amikacin, Tobramycin | 100 mM sodium tetraborate + 20 mM sodium deoxycholate + 15 mM beta-cyclodextrin, pH – 10 voltage: + 20kV, temperature: 25°C |
| Tetracyclines | Tetracycline, Oxytetracicline, Democlocycline, Chlortetracycline, Doxycicline, Minocycline | 15 mM ammonium acetate + 20 mM SDS, pH – 6.5 voltage: + 15kV, temperature: 25°C |
| Sulfonamides | Sulfamethazine, Sulfamerazine, Sulfathiazole, Sulfachloropyridazine, Sulfamethoxazole, Sulfacarbamide, Sulfaguanidine | 13.32 mM disodium hydrogen phosphate, 6.67 mM potassium dihydrogenphosphate + 40 mM SDS, pH – 7.5 voltage: + 21kV, temperature: 25°C fluorescence detection |
| Fluoroquinolones | Norfloxacin, Ciprofloxacin, Ofloxacin, Enrofloxacin, Danofloxacin | 25 mM sodium carbonate + 100 mM SDS, pH – 9.2 voltage: + 20kV, temperature: 30°C, UV detection 280 nm |
| Antifungal azoles | Fluconazole, Voriconazole, Itraconazole, Posaconazole | 25 mM phosphoric acid + 100 mM SDS + 13 % acetonitrile + 13% tetrahydrofuran, pH – 2.2 |
| Barbiturates | Phenobarbital, Amobarbital, Pentobarbital, Secobarbital, Butabarbital | 10 mM sodium tetraborate + 10 mM disodium hydrogenophosphate + 100 mM SDS + 15% acetonitrile. pH - 8.5 voltage: + 20 kV, UV detection 214 nm |
| Benzodiazepines | Flunitrazepam, Diazepam, Midazolam, Clonazepam, Bromazepam, Temazepam, Oxazepam, Lorazepam | 25 mM phosphate/borate + 75 mM SDS, pH – 9.3 |
| Phenotiazines | Promethazino, Ethopropazine, Trimeprazine, Methoprimeprazine, Thioridazine | 80 mM citric acid + 10 mMtetradecyltrimethylammonium bromide + 7 mM β-CD (9 mM HP β-CD), pH – 3.5 voltage: + 20kV, temperature: 25°C, UV detection 254 nm enantiomer separation |
| Tricyclic antidepressants | Imipramine, Amitriptyline, Desipramine, Nortriptyline, Doxepin, Trimipramine | 37.5 mM phosphate + 25 mMdodecyltrimethylammonium bromide + 2 M urea, pH – 8 voltage: + 25kV |
| Benzodiazepines | Alprazolam, Bromazepam, Chlordiazepoxide, Diazepam, Flunitrazepam, Medazepam, Oxazepam, Nitrazepam | 25 mM sodium tetraborate + 50 mM SDS + 12% methanol, pH – 9.3 voltage: + 25kV, temperature: 20°C, UV detection |
| Xanthines | Caffeine, Theobromine, Theophylline, Pentoxifylline | 20 mM sodium tetraborate + 100 mM SDS, pH – 9.3 voltage: + 30kV, temperature: 25°C, UV detection 274 nm |
Table 2: Applications of MEKC in the analysis of different pharmaceutical substances.
Another application of MEKC is the chiral separation of optically active pharmaceutical substances. Enantiomer separation by MEKC involves the addition of a chiral agent such as chiral surfactants, crown ethers, or CDs to the background electrolyte with chiral/achiral micelles. Chiral MEKC with chiral surfactants is an important separation mode for chiral compounds, with chiral surfactants including also naturally occurring compounds such as bile salts, amino acids or glucose. Chiral separation in MEKC is affected by the affinity of the enantiomers toward the micelles, and the concentration of the micellar phase, which depends on the aggregation properties of the chiral surfactants. MEKC can be used for the separation of structural related impurities from the main active drug, and has been proven an alternative to HPLC for quantitation of compounds and the determination of drugrelated impurities. The structurally related impurities of a drug will possess similar structural and physico-chemical characteristics to the main component, which makes their separation and determination a challenging task. The high separation efficiencies possible for CE often allow a small degree of selectivity to provide an acceptable resolution. The separation and determination of drug-related impurities using CE has been extensively studied, and the method performance and validation data obtained clearly shows that CE methods are successful applications in this area.
MEKC is relatively new technique in chromatographic separation and has wider applications in pharmaceutical drug developmental and drugs ranging from combinatorial chemistry, chiral (Enantiomers), separation and purification, clinical fluid analysis. This technique has advantages over others as small sample and solvent requirements with high resolutions. The micelle formation by surfactants further advances the technique as capillary electrophoresis.