Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research - (2019)Volume 7, Issue 3
Aim: To demonstrate the ability of a probiotic supplement (BIOHM) to disrupt pathogenic gastrointestinal biofilms, thus enhancing nutrient absorption.
Methods: A filter insert model using Caco-2 cells to mimic an intestinal monolayer was employed to determine the ability of BIOHM to disrupt a mixed species (Candida tropicalis, Escherichia coli and Serratia marcescens) biofilm known to occur in patients with Crohn’s disease. Penetration of vitamin C and casein as representative of vitamins and proteins, respectively, was then measured.
Results: Application of BIOHM led to a significant increase in casein penetration through the Caco-2 cell epithelial monolayer in the absence of biofilms (p value<0.0001). Additionally, the combination of mixed species biofilms grown on a Caco-2 cell monolayer with BIOHM filtrate resulted in higher penetration of vitamin C and casein through the monolayer compared to untreated controls.
Conclusion: Our in vitro data indicates that the combination of ingredients in the BIOHM probiotic may enhance nutrient permeability, thus leading to increased overall absorption of proteins and vitamins.
Probiotics; BIOHM; Nutrient absorption; Biofilms
Technological advancements that have improved identification of the microbial makeup of the human gastrointestinal (GI) tract, as well as the biochemistry of digestion, have also increased our understanding of the mechanisms of nutrient absorption. Recently published works have identified many of the microbiome constituents of a balanced gut, as well as which imbalances (dysbiosis) have been associated with disease states such as gut inflammation [1-4]. Further, characterization of the action of both host and microbial enzymes has served to inform the absorption routes of nutrients through the intestine.
The interaction of gut microorganisms with the intestines begins at the mucosal-associated layer that covers the epithelium. This layer, consisting of enterocytes and mucin secreted by goblet cells, provides a habitat for beneficial gut flora [5]. Moreover, the microbial communities residing in our gut (microbiome) influence various functional activities including nutrient absorption, protection of mucosal surfaces, and structure and function of the gut [6]. Host and microbial enzymes within this layer are responsible for breaking down ingested food into micronutrients that can be absorbed through the intestinal wall. For example, proteins, or more specifically oligopeptides, must be hydrolyzed by aminopeptidases so that specific transport proteins can facilitate the absorption of amino acids across the “brush-border” membrane of the enterocytes [7]. Vitamins are also known to be absorbed through the intestinal wall of the small intestine, including riboflavin (vitamin B2) and ascorbic acid (vitamin C). However, disruption of the mucin layer may have deleterious side effects such as invasion by pathogenic organisms or development of metabolic disorders [8]. Recently, we showed that patients with Crohn’s disease have much higher levels of the fungus Candida tropicalis compared to their healthy family members, as well as two bacteria, Escherichia coli and Serratia marcescens. Furthermore, using a mouse model we showed that these three pathogenic organisms worked together to form robust biofilms on the gastrointestinal lining [9]. By forming a thick layer over the gut lining, these pathogenic biofilms may impede nutrient absorption, as well as divert these nutrients as a nutritional source for their survival. For example, studies have shown that E. coli residing in a mixed species biofilm scavenges sugars for their growth [10], thereby reducing nutrient availability. Thus, approaches that can restore the microbiome balance by targeting pathogenic biofilms also support improvement of nutrient absorption by facilitating the efficient flow of the nutrients through the gut.
In recent years, use of probiotics has been promoted to ameliorate the dysbiotic state by increasing the numbers of beneficial microorganisms in order to offset the effects of pathogenic species. However, few studies have addressed the question of how these probiotic strains might positively affect nutrient absorption. Moreover, none of the published studies evaluated the ability of probiotics to improve nutrient absorption by targeting digestive biofilms formed by pathogens. We hypothesize that probiotic (BIOHM) use will lead to increased nutrient absorption via elimination of mixed species biofilms formed by pathogenic bacteria and fungi. To test this hypothesis, we determined the ability of the probiotic strains that comprise BIOHM (Saccharomyces boulardii, Lactobacillus acidophilus, L. rhamnosus, and Bifidobacterium breve) in combination with the enzyme amylase, to enhance absorption of vitamin C and casein (as representatives of vitamins and proteins, respectively) using an in vitro intestinal epithelial cell monolayer model.
Nutrients tested
In this study we assessed the ability of BIOHM to improve the absorption of vitamins and proteins, using vitamin C and casein (Sigma-Aldrich. St. Louis. MO) as representatives, in an epithelial cell culture model in the presence or absence of polymicrobial biofilms.
Nutrient absorption assays
Filter-Insert model used to evaluate nutrient absorption:
Figure 1 is a schematic representation of the filter-insert experimental model used. Briefly, a human epithelial colorectal adenocarcinoma cell line (Caco-2) purchased from the American Type Culture Collection (ATCC, Manassas, VA) was seeded onto the apical side of a trans-well filter at a concentration of 1.5 × 105 cells/ml and allowed to form a confluent monolayer for 10 days. With proper culture conditions, Caco-2 cells can differentiate and polarize to phenocopy enterocytes that express tight junctions, microvilli, and enzymes and transporters that are generally associated with the small intestine. Next, C. tropicalis, E. coli, and S. marcescens were added to the cell monolayers at a concentration of 1 × 106 cells/ml (for each organism) for a total concentration of 3 × 106 cells/ml and incubated for 6 hours to allow formation of a mixed species biofilm on top of the intestinal epithelial cells.
Figure 1. Experimental schematic of the cell based Trans-well system used to evaluate nutrient absorption.
Ability of the probiotic to enhance the absorption of Vitamin C:
To determine whether the probiotic can increase the ability of vitamin C to penetrate the epithelial cell layer, combined BIOHM filtrate and normal growth media containing vitamin C (100 mM) were added to the apical chamber and incubated for 6 hours at 37°C. Control wells received normal growth media containing Vitamin C. An aliquot (1 mL) from the basal chamber of the filter insert was then removed and assayed for the amount of vitamin C that passed through the intestinal epithelial monolayer.
The amount of vitamin C that was able to be pass through the epithelial cell monolayer was assessed using an L-ascorbic acid assay kit (Abnova, Taipei Taiwan). Pass through samples were collected and added to a 96-well plate and detected using the kit per the manufacturer’s protocol. The plate was incubated at room temperature for 10 minutes and the optical density was measured at 570 nm using the BioTek Synergy HTX Multi-Mode Microplate Reader.
Ability of the probiotic to enhance the absorption of casein:
The ability of BIOHM probiotics to enhance protein penetration through a Caco-2 cell monolayer was determined using casein as a representative protein assessed via the in vitro intestinal epithelial cell monolayer model described above. Two different experimental conditions were used to evaluate the ability of BIOHM to affect casein absorption. In the first condition we assessed the ability of the probiotic to enhance casein absorption by the epithelial cells in the absence of a mixed species biofilm (control: untreated monolayer+casein only [1 mg/mL], and treated: BIOHM filtrate+monolayer+casein only [1 mg/mL]), while in the second experimental condition we assessed the ability of the probiotic to aid casein penetration through the intestinal epithelial cells previously overlaid with a mixed species biofilm (cell monolayer: untreated controls (casein [1 mg/mL]+biofilm), and BIOHM filtrate treated (casein [1 mg/mL]+biofilm). The Caco-2 cells were seeded onto the apical side of the transwell membrane for 10 days to form a confluent cell monolayer. The biofilm organisms were then added onto the cells and incubated for 2 hours to allow for biofilm adherence. Subsequently, BIOHM probiotic and casein protein were added to each respective well and incubated for an additional 6 hours. After incubation, samples were collected from the basal chamber of the plates to assess the amount of casein absorbed.
The amount of casein absorbed through the cell monolayer was quantitated using a Qubit® protein assay (ThermoFisher Inc., St. Louis, MO). Briefly, the Qubit 4 fluorometer was calibrated with 0, 200, 400 ng/ml casein standards. Next, 10 μl of sample collected from the basal chamber of the filter insert was added to a protein assay reagent and measured at room temperature.
One-way ANOVA with a Turkey post-hoc test was used to compare treated versus untreated casein absorption since we had more than 3 groups, while an unpaired t-test was used to compare treated and untreated samples with vitamin C. All tests were done in quadruplicate. A p value<0.05 was considered significant.
BIOHM treatment facilitated increased permeability of vitamin C
As shown in Figure 2, treatment of mixed species biofilms formed on a Caco-2 cell monolayer with BIOHM filtrate plus amylase resulted in increased vitamin C detection in the basal layer of the cell culture model compared to untreated controls (mean ± SE: 440.8 μM/ml ± 92.22 μM/ml and 187.3 μM/ml ± 54.55 μM/ml for BIOHM-treated and untreated biofilms, respectively, p value=0.05). This data indicates that BIOHM probiotic aids in vitamin permeability through the intestinal cell layer.
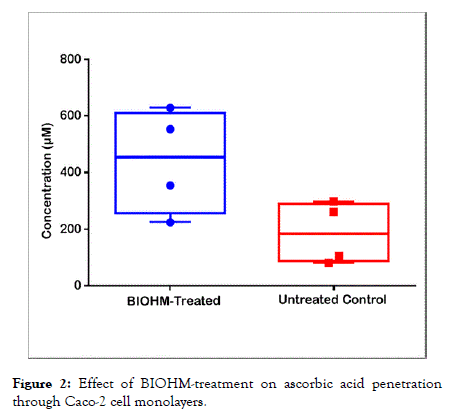
Figure 2. Effect of BIOHM-treatment on ascorbic acid penetration through Caco-2 cell monolayers.
BIOHM treatment led to higher permeability of casein
Table 1 summarizes the casein permeability findings. Treatment with the probiotic led to a significant increase in penetration of casein through the epithelial cell monolayer in the absence of biofilms (p<0.0001), where a 1.9-fold higher casein penetration was noted compared to untreated controls. This data indicates that BIOHM improved protein permeability through an intestinal cell model.
| Growth condition | Average amount of Casein Protein absorbed (µg/mL) ± SE | p value of BIOHM compared to untreated control |
|---|---|---|
| Untreated control+casein | 24.2 ± 0.5 | - |
| BIOHM treated+casein | 46.6* ± 1.0 | <0.0001 |
| Untreated control+biofilm+casein | 26.9 ± 2.9 | - |
| BIOHM treated+biofilm+casein | 42.4* ± 3.5 | 0.0008 |
Table 1: Effect of BIOHM treatment on casein absorption. Average amount of casein protein absorbed through Caco-2 cell monolayers expressed in µg/mL ± SE.
Additionally, our data showed that treatment with BIOHM led to a significant increase (p=0.0008) in casein permeability (1.58- fold higher absorption compared to untreated control) through an epithelial cell monolayer covered with a polymicrobial biofilm. This data indicates that the probiotic was able to facilitate nutrient permeability through epithelial cells despite the presence of a mixed species biofilm formed by pathogenic bacteria and fungi.
The effect of nutritional deficiencies on human health has been widely known since rickets and scurvy were first linked to a serious lack of vitamins D or C, respectively, in the diet. Today it is generally assumed that eating a diet containing lots of fruits and vegetables, while avoiding too many fats and sugar, will assure good health. Unfortunately, consumption of nutritious foods is not enough if these nutrients cannot be properly absorbed. Water-soluble vitamins, including thiamine, riboflavin, and ascorbic acid, are absorbed in the small intestine and require daily replenishment since they are readily excreted in the urine [11]. Similarly, complex carbohydrates require breakdown by enzymes produced in the brush-border membranes of enterocytes in the small intestine [8] Proteins must be broken down into amino acids or small peptides, a process that begins in the stomach and is completed by enzymes from the enterocyte membrane in the small intestine [7,12].
Thus, the importance of proper function of the small intestine cannot be overestimated. It stands to reason that any obstruction of the mucosal layer of the small intestine may impede the vital function of the enterocytes in nutrient absorption. In studying the effects of prebiotic inulin-type fructans on the microbial balance of the gut, Kleesen and Blaut discovered that these prebiotics actually change the architecture of the lining of the small intestine, resulting in a significantly thicker mucin layer [5]. Earlier experiments suggested that increased mucin thickness resulted in increased expression of brush border enzymes and nutrient transport systems [13]. However, in order for absorption to occur, partially digested food from the stomach must be in direct contact with this mucosal layer. Formation of pathogenic biofilms over the top of the mucus lining prevents this contact from occurring.
Our previous experiments showed that C. tropicalis, E. coli, and S. marcescens, significantly more abundant in Cohn’s disease patients, formed pathogenic polymicrobial biofilms (PMB) capable of initiating an inflammatory response when grown together in vitro [14]. Subsequently, we were able to demonstrate the formation of robust PMBs comprised of these 3 organisms on the gastrointestinal lining epithelium in a murine model [15]. In an effort to discover a mechanism to prevent pathogenic biofilm formation, we formulated a commercial probiotic, BIOHM, which contains the strains Saccharomyces boulardii, Lactobacillus acidophilus, L. rhamnosus, and Bifidobacterium breve, in combination with amylase.
In this study, we showed that BIOHM improves the permeability of nutrients through an epithelial cell monolayer that mimics the small intestine regardless whether or not the intestinal cell monolayer was covered by polymicrobial biofilms. The observation that BIOHM can enhance nutrient permeability through an epithelial cell monolayer that does not have biofilm formed on its surface is not surprising. A previous study by Borthakur et al. evaluated the effect of the probiotic bacteria L. acidophilus (one of the probiotic strains in BIOHM) on the function of intestinal electrolyte absorption and showed that L. acidophilus secretes extracellular molecule(s) that stimulate apical chloride/hydroxyl exchange activity, thereby aiding permeability [16]. Another study indicated that probiotics may have utility in combating malnutrition, and suggested that further studies of probiotics in malnutrition are warranted [17].
Although previous studies have shown that probiotics can improve permeability of some dietary nutrients through a human intestinal cell monolayer, our study is the first to show that BIOHM can facilitate nutrient permeability in the presence of a polymicrobial mixed species biofilm formed by pathogenic bacteria and fungi. Several mechanisms may participate in this process whereby BIOHM improves nutrient permeability through an epithelial cell monolayer. Individually, the selected probiotic strains that comprise BIOHM each produce specific by-products with anti-biofilm properties. Murzyn et al. showed that S. boulardii secretes active compounds (e.g. capric acid [C10:0]) that inhibit different Candida virulence factors, including adherence and filamentation, both of which are critical for the ability of this yeast to form biofilms [18]. Furthermore, metabolites released by Lactobacillus species, such as sodium butyrate, have been shown to inhibit biofilm formation. In that regard, an in vitro study by Ribeiro et al. showed that L. rhamnosus cells and supernatant can reduce C. albicans biofilm formation [19]. Furthermore, Taraszkiewicz et al. showed that amylase disrupted biofilm formation by means of biofilm-enzyme degradation [20]. Interestingly, in separate experiments we showed that the neither the probiotic strains (S. boulardii, L. acidophilus, L. rhamnosus, and B. breve) when used singly, nor amylase were able to inhibit biofilms formed by the three pathogenic fungi and bacteria (data not shown). In contrast, the combination of these probiotic strains in the BIOHM formulation demonstrated dramatic changes in polymicrobial biofilms during co-culture experiments, including altered biofilm architecture and significant reduction of biofilm thickness. These changes are due in part to the prevention of Candida germination (formation of hyphae), an important candidal virulence factor. Formation of hyphal filaments is the means by which Candida is able to form a dense matrix of biofilm, in turn providing protection for pathogenic bacteria against antibacterial agents [21]. These dense biofilms also prevent partially digested food from coming into contact with the brush-membranes of the intestinal lining, thus reducing absorption of nutrients.
Our findings demonstrate that BIOHM enhances permeability of vitamin C and casein (representative of vitamins and proteins) in intact (healthy) and imbalanced (biofilm attached) epithelial monolayers via different mechanisms. The novel combination of organisms and amylase in BIOHM shows great promise in disrupting pathogenic biofilms on gastrointestinal surfaces (i.e., the gut), thereby facilitating increased nutrient penetration and increased overall absorption of vitamins and proteins, thereby promoting increased gut health.
Citation: Ghannoum M, Ghannoum A, Long L, Sun PL, Isham N, McCormick TS (2019) The Probiotic BIOHM Improves Nutrient Absorption by Disrupting Gastrointestinal Biofilms. J Prob Health. 7:213. DOI: 10.35248/2329-8901.19.7.213
Received: 23-Aug-2019 Accepted: 23-Sep-2019 Published: 30-Sep-2019 , DOI: 10.35248/2329-8901.19.7.213
Copyright: © 2019 Ghannoum M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.