
Journal of Clinical Trials
Open Access
ISSN: 2167-0870

ISSN: 2167-0870
Research Article - (2020)
Objective: To investigate the efficacy of gluten-restricted diet for the disease activity of Rheumatoid Arthritis (RA) and clinical factors corresponding to the response.
Methods: Sixty three patients with active RA were included in this study. At baseline, we gave the patients the information about gluten-free and-contained foods, and asked them to refrain from the daily gluten consumption during the experimental period.
Results: The 16-week gluten-restricted diet significantly improved DAS28-CRP and CDAI scores. The percentages of patients achieving DAS28-CRP- and CDAI-defined remission or LDA were significantly increased at Week 16. When the self-reported levels about adherence to the gluten-restricted diet were divided into two categories, a significant improvement in DAS28- CRP, CDAI, and EULAR treatment response after 16 weeks was detected only in the patient group with the strict adherence to gluten-restriction. Significantly lower levels of Anti-Cyclic Citrullinated Peptide Antibodies (ACPA) were detected in responders to the gluten-restriction than in non-responders.
Conclusion: This study demonstrates that strict adherence to gluten-restricted diet results in decreased disease activity of RA patients with low ACPA.
Gluten; Rheumatoid arthritis; CDAI; DAS28-CRP; Anti-citrullinated peptide antibodies
Rheumatoid arthritis (RA) is an autoimmune inflammatory disease of unknown etiology and is characterized by persistent and chronic joint inflammation which markedly reduce the quality of life of the patients [1,2]. With undefined pathogenesis, previous studies reported a blend of genetic and environmental factors responsible for full expression of RA [3,4]. It has been reported that there are several risk factors contributing to the initiation and promotion of this complex disorder, such as age, gender, hormonal levels, cigarette smoking, alcohol, obesity, and dietary habits [5]. Identifying preclinical RA has taken on new importance because of multiple pharmacologic prevention trials that are underway, and these factors may be used in prediction models for future RA and to identify targets for prevention [5,6].
Diet has been suggested to influence the inflammatory activity levels in RA as an initiator and a risk factor for the development because micronutrients in the diet has been shown to influence the intestinal and systemic immune responses [7-11]. However, there is much less evidence of a role for nutritional factors in the pathogenesis of RA [12].
Our preliminary experiments indicated that patients with RA are more likely to prefer noodles than control patients (Tani et al. unpublished observation). Gluten is an important ingredient of wheat protein, as about 90% of the total protein content is gluten [13]. Besides, it is also present in barley, oat and rye, and these gluten-rich cereals constitute the major part of our diet such as noodles, pasta, bread, and snacks [14]. Gluten is a water-insoluble protein, and induces epithelial cell damage within the small bowel, which is considered to trigger immune reactions in gluten sensitive diseases such as celiac disease [14,15]. Interestingly, patients with celiac disease exhibit increased levels of Rheumatoid Factor (RF) in the gut mucose and to be at increased risk for RA [16]. However, the direct role of the gluten exposure in the development of autoimmune conditions associated with RA is still a matter of debate.
A few previous studies demonstrated the effect of gluten-free diet on the disease activity of RA [17,18]. However, the actual effect of gluten-free diet is still unknown because the studies represented combined effects of vegetarian diet and gluten-free diet. The primary aim of this study is to investigate whether gluten-restricted diet could yield an improvement in the activity of patients with active RA comparing the effects among the levels of adherence to gluten-restriction and to clarify clinical factors to affect the response to the gluten-restricted diet.
Study design and patients
The present study was conducted at Tokushima Prefectural Kaifu Hospital and Toyo Hospital which had a professional department for RA medical treatment. RA was diagnosed according to the 1987 American College of Rheumatology classification criteria for RA [19] or ACR/EULAR 2010 RA criteria [20]. Patients were allowed to continue on daily doses of non-steroidal anti-inflammatory drugs (NSAIDs), oral glucocorticoids and conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), biological DMARDs (bDMARDs) and targeted synthetic DMARDs (tsDMARDs). Patients who either started or discontinued these medications for the previous 8 weeks and during the study were excluded. All medications except NSAIDs were on the same dosage. Patients who already lived on a gluten-free diet due to the gluten allergy were excluded. Between October 2019 and January 2020, 109 patients with RA who had been treated in the hospitals were enrolled in this study. Thirty five patients were excluded from this study because their disease activities in RA at baseline were in remission. During the study, 11 patients were removed from this study; eight patients refused to continue the diet, a patient complicated pneumonia, patients admitted due to bone fracture, and a patient needed bDMARDs changed. Finally, 63 patients with RA completed this study.
All patients gave written informed consent before participating in the study. The present study was approved by the Tokushima University Hospital ethics committee and was performed in accordance with the Declaration of Helsinki.
Pathogenesis
We informed the patients the experimental protocol. Before baseline (Week 0), all patients had been in omnivorous diet. At baseline, to patients who agreed with the study we explained what gluten-free and -contained foods were, and asked to refrain from the daily gluten consumption during the experimental period. Since gluten is included in wheat, rye, and barley, the patients were informed to avoid food products derived from these cereals, such as bread, pasta, cakes and biscuits. Other wheat-based products, which were common foods in Japan such as Japanese noodles, Japanese Tempura, cutlet, and jiaozi, were recommended to avoid taking. On the other hand, naturally gluten-free foods such as rice, potatoes, vegetables, fruit, unprocessed meat and fishes could be accepted. Manufactured gluten-free versions of wheat-based foods, such as breads, pasta and cereals were included in gluten- free foods. This designing study was considered to be nutritionally complete. The patients lived in gluten-restricted diet for 16 weeks. We did not strictly prohibit taking any gluten-contained foods when the patients strongly wanted to consume them. At Week 16, the patients were questioned about the level of subjective adherence to the gluten-restricted diet. The self-reported levels about adherence to the gluten-restricted diet during 16 weeks were divided into six categories; “complete”, “excellent”, “moderate”, “fair”, “poor”, and “not at all”. Using the information, the levels of gluten-restricted diet of each patient were divided into two groups; “complete”, “excellent” and “moderate” were divided into Strong Gluten-restricted Diet (SGD), “fair”, “poor” and “not at all” into Light Gluten-restricted Diet (LGD). There were no significant differences between SGD and LGD groups in background data such as age, age at onset, disease duration or sex, and in baseline clinical data; Anti-Citrullinated Peptide Antibodies (ACPA), RF, C-reactive Protein (CRP), the Disease Activity Score assessing 28 joints with CRP (DAS28-CRP) or Clinical Disease Activity Index (CDAI) (data not shown).
Data collection for disease activity
The following disease parameters were recorded at baseline and Week 16: The 28 tender and swollen joint counts (TJC28 and SJC28) and patient (PtGA) and physician (phGA) global assessment of disease activity [mm] in which 0=best and 100=worst [21]. The following laboratory data were assessed: CRP, RF, and ACPA. Clinically, Cyclic Citrullinated Peptides (CCP) can be used as test substrates for detecting ACPA. We did not have records on ACPA when cases were diagnosed prior to its widespread use. The abnormal levels of RF, CRP and ACPA were defined as >20 IU/ml, >0.3 mg/dl, and > 4.5 U/ml, respectively. When data of RF, CRP, and ACPA were under the sensitivity level, data of the detection limit were used (RF=4 IU/ml, CRP=0.05 mg/dl, anti-CCP antibodies=0.5 U/ml). As established definitions in the evaluation of RA disease activity, DAS28-CRP and CDAI were used in this study. DAS28-CRP was scored, using TJC28, SJC28, ptGA, and CRP [22]. CDAI was scored as: TJC28+SJC28+PtGA+PhGA [23]. Concerning DAS28-CRP, the patients were divided into those in remission (<2.3), and those in non-remission (low disease activity; LDA, >2.3 and <2.7: moderate disease activity; MDA, 2.7-4.1: High Disease Activity, HDA, >4.1). About CDAI, the patients were divided into those in remission (<2.8), and those in non-remission (LDA, >2.8 and<10: MDA, 10-22: HDA, >22).
Definitions of clinical assessment
Mean rates of remission, LDA, MDA, and HDA in DAS28-CRP and CDAI were calculated and compared at baseline and Week 16. Clinical responses were defined by the European League Against Rheumatism (EULAR) response criteria [24]. Good response: DAS28-CRP improvement >1.2 in present DAS28-CRP <2.7.Moderate response: DAS28-CRP improvement >0.6 and 1.2 in present DAS28-CRP < 2.7, and >2.7 and <4.1; DAS28- CRP improvement >1.2 in present DAS28-CRP >4.1, and 2.7 and <4.1. No response: DAS28-CRP improvement < 0.6, and DAS28-CRP improvement >0.6 and <1.2 in present DAS28-CRP >4.1. Changes in DAS28-CRP and CDAI scores at Week 16 from baseline data were analyzed and were expressed as ΔDAS28-CRP and ΔCDAI, respectively.
Statistical analysis
Median values were calculated for non-normally distributed continuous variables. Data were mainly presented as median and 25th-75th quarter, and count and percentage. Comparison between independent means was made using Mann-Whitney U test or Kruskal-Wallis test. Longitudinal changes of each parameter before and after the diet were examined by the Wilcoxon signed-rank test or Friedman test. The relationship between categorical variables was evaluated by the chi-square test. The results were regarded as significant when p value was <0.05. All statistical analyses were performed using IBM SPSS statistics version 24 software (Chicago, IL, USA).
Patient baseline characteristics
A total of 63 patients completed 16 weeks on the diet regimen and were included in our analyses. Table 1 shows the basement characteristics of overall patients. Out of the overall patients, 49 (78%) were female and the median age was 67 years old. The median disease duration of RA since diagnosis was 9 years. During this study, TJC, SJC, PtGA, PhGA and CRP were measured in all patients. RF at baseline and ACPA at diagnosis of RA were measured in 58 patients (92.1%) and 40 patients (63.5%), respectively.
| Variables | |
|---|---|
| Sex (male : female) | 14 (22%): 49 (78%) |
| Age (y) | 67 (56 -75) |
| Disease duration (y) | 9 (5-14) |
| TJC, 0-28 | 1 (1-4) |
| SJC, 0-28 | 3 (2-5) |
| PtGA (mm) | 22 (12-33) |
| PhGA (mm) | 27 (17-37) |
| ACPA (Anti-CCP antibodies) (U/ml)* | 95.5 (12.0-260.3) |
| RF (IU/ml) ** | 40.0 (8.0-88.5) |
| CRP (mg/dl) | 0.15 (0.05-0.43) |
| DAS28-CRP | 3.1 (2.6-3.3) |
| CDAI | 11.1 (7.5-15.1) |
Values are presented as number (%) and median (interquartile rage). TJC: Tender Joint Counts; SJC: Swollen Joint Counts, PtGA: Patient Global Assessment of disease Activity; PhGA: Physician Global Assessment of disease Activity; ACPA: Anti-cyclic Citrullinated Peptide Antibodies; CCP: Cyclic Citrullinated Peptide; RF: Rheumatoid Factor; CRP: C-Reactive Protein; DAS28-CRP: Disease Activity Score assessing 28 joints with CRP; CDAI: Clinical Disease Activity Index. *n=40 (63.5%), **n=58 (92.1%).
Table 1: Patient baseline characteristics.
Efficacy of the low gluten diet in overall patients
Table 2 shows the changes in clinical variables associated with disease activity of overall patients with RA. TJC28 and SJC28 were significantly decreased at Week 16 when compared with that at baseline. The level of PhGA was significantly improved at Week 16. There were no significant changes in the levels of PtGA. Concerning hematological data, no significant decrease of RF or CRP was detected at Week 16. Of the composite measures, DAS28-CRP and CDAI were significantly improved at Week 16 when compared with that at baseline.
| Variables | Baseline | Week 16 | p value |
|---|---|---|---|
| DAS28-CRP | 3.1 (2.6-3.3) | 2.7 (2.2-3.3) | <0.001 |
| CDAI | 11.1 (7.5-11.1) | 7.8 (5.7-14.5) | <0.001 |
| TJC 28 | 3 (1-4) | 2 (0-3) | 0.001 |
| SJC 28 | 3 (2-5) | 2 (1-5) | 0.022 |
| PtGA (mm) | 22 (12-33) | 22 (11-36) | 0.689 |
| PhGA (mm) | 27 (17-37) | 17 (10-28) | <0.001 |
| RF (IU/L) | 40.0 (8.0-88.5) | 39.5 (9.3-101.3) | 0.649 |
| CRP (mg/dL) | 0.15 (0.05-0.43) | 0.14 (0.05-0.49) | 0.891 |
Values are presented as median (interquartile rage). DAS28-CRP; Disease Activity Score assessing 28 joints with CRP, CDAI: Clinical Disease Activity Index; TJC: Tender Joint Counts; SJC: Swollen Joint Counts, PtGA: Patient Global assessment of disease Activity, PhGA: Physician Global assessment of disease Activity, RF: Rheumatoid Factor; CRP: C-Reactive Protein.
Table 2: Changes of clinical parameters in overall patients.
Comparison in efficacy among levels of adherence to glutenrestricted diet
To evaluate the effect of gluten-restriction on the disease activity of RA, patients were divided by the levels of gluten-restricted diet into two categories; SGD and LGD, and the longitudinal changes in the disease activities were compared between them (Table 3). Of 63 patients, 41 (65%) were included in SGD and 22 (35%) in LGD. Of the changes in composite measures, the level of DAS28-CRP and CDAI was significantly decreased in patients with SGD but not in those with LGD at Week 16 when compared with that at base line. A significant decrease was detected in TJC28, SJC28 and PhGA in patients only of SGD group. There were no significant decreases in biological data; RF or CRP in SGD or LGD.
| Variables | Gluten-restriction | Baseline | Week 16 | p value |
|---|---|---|---|---|
| DAS28-CRP | SGD | 2.94 (2.54-3.29) | 2.37 (1.86-3.04) | <0.001 |
| LGD | 3.22 (2.72-3.75) | 3.12(2.58-3.88) | 0.485 | |
| CDAI | SGD | 10.90 (6.75-13.55) | 7.00 (4.55-11.85) | <0.001 |
| LGD | 11.55 (8.38-16.30) | 8.40 (6.78-16.35) | 0.259 | |
| TJC 28 | SGD | 2.0 (1.0-4.0) | 1.0 (0.0-2.5) | <0.001 |
| LGD | 3.0 (1.0-5.3) | 2.0 (1.0-4.0) | 0.515 | |
| SJC 28 | SGD | 3.0 (2.0-5.5) | 2.0 (1.0-4.0) | 0.002 |
| LGD | 3.5 (1.8-5.3) | 3.0 (2.0-5.0) | 0.962 | |
| PtGA | SGD | 2.2 (1.2-3.3) | 2.0 (1.3-3.1) | 0.821 |
| LGD | 2.2 (1.0-3.6) | 2.2 (0.9-4.5) | 0.313 | |
| PhGA | SGD | 2.2 (1.7-3.7) | 1.5 (0.8-2.2) | <0.001 |
| LGD | 2.8 (2.0-4.2) | 1.9 (1.4-3.9) | 0.13 | |
| RF | SGD | 31.0 (7.0-69.5) | 35.0 (9.0-70.0) | 0.089 |
| LGD | 40.0 (8.5-129.0) | 44.0 (9.5-120.0) | 0.185 | |
| CRP | SGD | 0.14 (0.07-0.24) | 0.08 (0.05-0.23) | 0.758 |
| LGD | 0.42 (0.05-1.35) | 0.40 (0.05-1.10) | 0.868 |
Values are presented as median (interquartile rage). DAS28-CRP: Disease Activity Score assessing 28 joints with CRP; CDAI: Clinical Disease Activity Index; TJC: Tender Joint Counts; SJC: Swollen Joint Counts; PtGA: Patient Global assessment of disease Activity; PhGA: Physician Global assessment of disease Activity; RF: Rheumatoid Factor; CRP: C-Reactive Protein, SGD: Strong Gluten-restricted Diet; LGD: Light Gluten-restricted Diet.
Table 3: Clinical parameter related to disease activity of RA comparing between the levels of gluten-restriction.
We next examined the changes of DAS28-CRP (ges of DAS28-CRP (ΔDAS28-CRP) and CDAI (ΔCDAI) of each patient from baseline to Week 16 (Figure 1). A significant improvement in ΔDAS28- CRP was DAS28-CRP) and CDAI (ΔCDAI) of each patient from baseline to Week 16 (Figure 1). A significant improvement in DAS28-CRP was detected in overall patients [-0.27 (-0.97, 0.09), median (interquartile range)]. When the level of gluten restriction was divided into 2 groups, the improvement was detected in patients with SGD [-0.39 (-1.12, -0.02)] but not in those with LGD [-0.10 (-0.48, 0.36)] (Figure 1A). A significant improvement in ΔCDAI was also detected in overall patients [-2.10 (-5.40, 1.10)], and when the level of gluten reduction was divided into 2 groups, the improvement was detected in patients with SGD [-3.00 (-6.00, 0.80)] but not in those with LGD [-1.15 (-3.20, 1.98)] (Figure 1B).
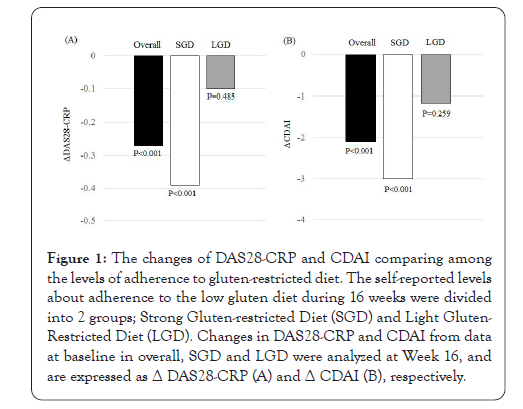
Figure 1: The changes of DAS28-CRP and CDAI comparing among the levels of adherence to gluten-restricted diet. The self-reported levels about adherence to the low gluten diet during 16 weeks were divided into 2 groups; Strong Gluten-restricted Diet (SGD) and Light Gluten- Restricted Diet (LGD). Changes in DAS28-CRP and CDAI from data at baseline in overall, SGD and LGD were analyzed at Week 16, and are expressed as Δ DAS28-CRP (A) and Δ CDAI (B), respectively.
Figure 2 shows the changes in percentages of disease activities determined by DAS28-CRP and CDAI scores. The proportion of patients achieving DAS28-CRP-defined remission or LDA was 31.7% of patients at baseline, which was significantly increased to 54.0% at at Week 16 (Figure 2A). Concerning CDAI disease activity, 39.7% of patients were in remission or LDA at baseline, which was significantly increased to 61.9% at Week 16 (Figure 2B). We next examined the EULAR treatment response using DAS28- CRP score (Figure 3). A good or moderate EULAR response was observed in 36.5% of RA patients at Week 16. There was significantly more percentage of patients with a good or moderate EULAR response at Week 16 in patients with SGD (46.3%) than in those with LGD (18.2%).
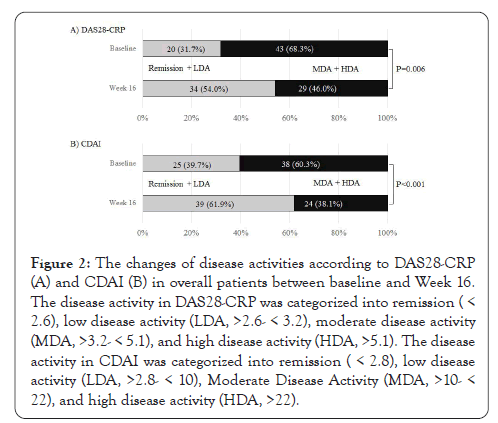
Figure 2: The changes of disease activities according to DAS28-CRP (A) and CDAI (B) in overall patients between baseline and Week 16. The disease activity in DAS28-CRP was categorized into remission( < 2.6), low disease activity (LDA, >2.6- < 3.2), moderate disease activity (MDA, >3.2- < 5.1), and high disease activity (HDA, >5.1). The disease activity in CDAI was categorized into remission (< 2.8), low disease activity (LDA, >2.8- < 10), Moderate Disease Activity (MDA, >10- < 22), and high disease activity (HDA, >22).
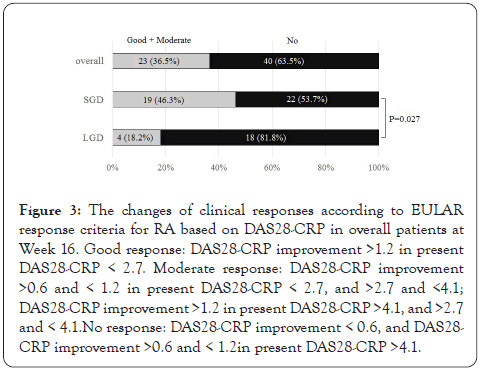
Figure 3: The changes of clinical responses according to EULAR response criteria for RA based on DAS28-CRP in overall patients at Week 16. Good response: DAS28-CRP improvement >1.2 in present DAS28-CRP < 2.7. Moderate response: DAS28-CRP improvement >0.6 and < 1.2 in present DAS28-CRP < 2.7, and >2.7 and <4.1; DAS28-CRP improvement >1.2 in present DAS28-CRP >4.1, and >2.7 and < 4.1.No response: DAS28-CRP improvement < 0.6, and DAS28- CRP improvement >0.6 and < 1.2in present DAS28-CRP >4.1.
The relation of responses to clinical parameters
We examined clinical parameters which were ralated to the EULAR treatment response using DAS28-CRP score (Table 4). Significantly lower levels of ACPA at diagnosis were detected in responders than in non-responders. There were no differences between responders and non-responders in age, disease duration, sex, RF or CRP. No differences were detected in the disease activities at baseline such as DAS28-CRP, CDAI, TJC, SJC, Pt-GA, Ph-GA between responders and non-responders.
| Variables | Responders (n=23) | Non-responders (n=40) | p value |
|---|---|---|---|
| Age at onset | 57.0 (48.3-63.5) | 57.0 (51.0-70.0) | 0.732 |
| Age at baseline | 60.5 (57.8-69.8) | 64.0 (54.5-78.5) | 0.507 |
| Disease duration | 4.0 (2.0-8.0) | 7.0 (3.5-9.0) | 0.632 |
| Sex (M/F) | Jul-16 | 7/33 | 0.234 |
| ACPA at diagnosis | 24.8 (0.6-102.2) | 100.0 (29.6424.7) | 0.029 |
| RF at diagnosis | 34.4 (4.8-69.6) | 35.0 (68.0-139.8) | 0.782 |
| RF at baseline | 15.5 (5.8-55.5) | 40.0 (6.5-236.5) | 0,268 |
| CRP at baseline | 0.15 (0.08-0.31) | 0.15 (0.06-0.64) | 0.517 |
| DAS28-CRP at baseline | 3.0 (2.5-3.3) | 3.1 (2.6-3.5) | 0.808 |
| CDAI at baseline | 10.4 (6.7-13.1) | 12.3 (6.5-15.7) | 0.549 |
| TJC at baseline | 3.0 (1.0-4.0) | 2.0 (1.0-4.0) | 0.216 |
| SJC at baseline | 3.0 (1.0-4.0) | 3.5 (2.0-6.0) | 0.299 |
| Pt-GA at baseline | 2.1 (1.2-2.8) | 2.2 (1.2-3.7) | 0.663 |
| Ph-GA at baseline | 2.2 (1.6-3.1) | 2.8 (1.7-3.8) | 0.395 |
Values are presented as median (interquartile rage). ACPA: Anti-cyclic Citrullinated Peptide Antibodies; RF: Rheumatoid Factor; CRP: C-Reactive Protein; DAS28-CRP: Disease Activity Score assessing 28 joints with CRP; CDAI: Clinical Disease Activity Index; TJC: Tender Joint Counts, SJC: Swollen Joint Counts; PtGA: Patient Global Assessment of disease Activity; PhGA: Physician Global assessment of disease Activity.
Table 4: Comparison of background factors between responders and non- responders.
This study demonstrates that 16-week strict adherence to gluten- restricted diet reduces the disease activity of active RA. When the extent of gluten-restriction in the diet was divided into two levels, SGD (strong) and LGD (light), the efficacy was observed only in patients with SGD. In this study, we showed that gluten-restricted diet reduces the disease activity of active RA patients with at LDA or more, and the effect was significantly higher in RA patients with low ACPA than in those with high ACPA.
The level of ACPA was significantly lower in responders to the gluten-restricted diet than in non-responders, suggesting that gluten-restriction may be effective for RA patients with low levels of ACPA. Two autoantibodies, ACPA and RF, are known and clinically used to examine the diagnosis and prognosis of RA [25,26]. The present study showed that the significant inverse relation to the responsiveness to the gluten-restricted diet is found in ACPA but not in RF. RA has been shown to consist of two different disease subsets based on the presence of ACPA, seropositive or seronegative [27]. However, the difference in the development of RA between ACPA-positive and ACPA- negative RA is still unknown. Recent studies have examined the role of ACPA, autoantibodies for citrullinated proteins, in the pathogenesis of RA [28]. Protein citrullination is an essential step in the initiation of the autoimmune response in RA [29,30]. The interaction of citrullinated proteins and ACPA has an important role in inducing activation of inflammatory cells and cytokine production which contribute the inflammatory and destructive response of RA joints [31]. The seropositivity, positive ACPA, is associated with more phenotypic severity of RA joint inflammation [28]. On the other hand, there is little information about the mechanism of the development of ACPA-negative RA. This study suggests that gluten may be responsible for the pathogenesis for ACPA-negative RA because gluten-restricted diet is more effective in RA patients with low ACPA than in those with high ACPA.
The mechanism by which gluten-restricted diet reduces the disease activity of RA is unclear. Since gluten is a water-insoluble protein, large gluten peptides interact with the immune system, affect the intestinal permeability, and modify the gut microbial activity in the small instestine [32]. Moreover, gluten intake is a well-known triggering antigen that initiates immune reactions against small bowel cells in gluten sensitive diseases such as celiac disease [13,32]. Similary, the gut may have a primary role in the inflammatory process in RA, and the change of the intestinal microflora and its metabolism may significantly contribute to the diminution of the rheumatoid symptoms [10]. Interestingly, patients with celiac disease have been shown to be associated with RA [16,33,34]. Increased prevalence of RF positivity in patients with celiac diseases indicates that patients with celiac disease might be at a higher risk of developing RA [16]. Inversely, patients with autoimmune diseases have a significantly higher prevalence of celiac disease than the healthy population [33]. These facts that there are several similarities in the clinical findings between celiac disease and RA suggest that gluten may be responsible for the pathogenesis of a certain proportion of patients with RA. Gluten- free diet is associated with reduced auto-inflammatory process and altered inflammatory cytokine response [10,35]. Strict adherence to gluten-free diet prevents the development of further autoimmune diseases in patients with celiac disease [8], and early exposure to gluten results in modifying the immunological response [33,35- 38]. These results suggest that immunological abnormalities in gastrointestinal tract due to gluten may be responsible for not only celiac disease but also RA.
This study showed that gluten-restricted diet significantly improved composite measures concerning the activity of RA; DAS28-CRP and CDAI. Of the separate disease activity measures within DAS28- CRP and CDAI, gluten-restricted diet significantly improved joint symptoms, TJC, SJC and PhGA. However, it did not affect serum levels of CRP or RF. These results suggest that gluten-restricted diet seems to lead to the improvement of joint symptoms associated with RA but not to the laboratory tests. Concerning CRP, the previous two studies investigated the change during gluten-free vegan diet [17,18]. A significant decrease in CRP with higher response rate at ACR20 is observed in RA patients with gluten free vegan diet after 1 year [17]. Similary, gluten-free vegan diet reduces CRP at 12 months but not at 3 months though DAS28 is decreased since 3 months [18]. These reports suggest that a decrease in CRP may need longer period diet such as one year than a period examined in the present study.
There are several limitations to this study. Firstly, since this study was not double blind, the patients inevitably knew the level of gluten-restricted diet in themselves. Since the level of adherence to gluten-restricted diet was recorded by the patients subjectively but not objectively, it was difficult to avoid the pose of the patients in the study. Secondly, it is difficult for patients to be in diet with complete avoidance of gluten because plenty of products contain hidden gluten, such as soups, sausages, soy sauce and ice cream, and moreover there are traces of gluten even in gluten-free labeled products. Therefore, in this study, we did not use gluten-free patient group and control group, and compared results between two groups by the levels of gluten-restriction. We have a plan of a case-control study using control patients being in omnivorous diet.
This study showed that the gluten-restricted diet reduces the disease activity of RA. RA is associated with a high health-care burden due to the expensive medical treatment, and causes significant costs for society due to the increased use of healthcare resources Especially, costs of bDMARDs and tsDMARDs for RA are in remarkably high though these medications have proven to be an effective treatment for this disorder to it is possible that gluten- restricted diet may have a role in reducing the basic activity of RA resulting in beneficial effect in cost performance of therapeutic strategy of this disorder. Further longer time studies are needed to clarify the beneficial efficacy of the gluten-restricted diet on the disease activity and joint prognosis of RA.
The authors declare that they have no conflict of interest to declare.
The authors thank Ms. Yayoi Tagawa for her valuable secretarial support.
Citation: Tani K, Okura Y, Kawaminami S, Kawahito K, Kondo K, Takahashi R, et al. (2020) Strict Gluten-Restricted Diet Reduces Disease Activity of Rheumatoid Arthritis with Low Anti-Cyclic Citrullinated Peptide Antibodies. J Clin Trials. 10:441.
Received: 27-Nov-2020 Accepted: 09-Dec-2020 Published: 16-Dec-2020
Copyright: © 2020 Tani K, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.