Autism-Open Access
Open Access
ISSN: 2165-7890
ISSN: 2165-7890
Research Article - (2020)Volume 10, Issue 2
The existence of morphological and physiological abnormalities in the autism brain was recognized in the 1960s. Today, in view of many such extensive pathological findings, it has been stated and reiterated that autism spectrum disorder (ASD) has a biological rather than a psychological basis. Despite this, other than pharmacologic agents that address limited aspects of symptoms, development of therapies for ASD has been focused primarily on psychosocial measures based on principles of applied behavioral analysis. With this latter approach, however, a generally accepted intervention or group of interventions which consistently and comprehensively address the needs of most individuals with autism in a cost-effective and time-efficient manner has not yet been established.
While examining reports of another medical intervention, hyperbaric oxygen therapy with inspired oxygen partial pressures typically ranging from 0.31 to 1.5 atm. for neurological conditions such as autism, we wondered if increased oxygen partial pressure, alone, without the encumbrance, risk, and cost of hyperbaric exposure would produce useful clinical outcomes. In five pilot cases, therefore, a normobaric hyperoxic treatment we have called Microbaric® Oxygen Therapy (MBO2) was administered to preteen and teenage boys with ASD. All results were positive, some remarkably so, and addressed the full scope of the subject’s symptoms. Also, follow-up for over seven years in several cases suggests these outcomes are permanent.
To help encourage controlled research on MBO2 for ASD, we have laid out a hypothetical basis for its efficacy using our own pilot study outcomes and observations together with relevant information from the scientific literature, the most important of which we believe relates to regional brain hypoperfusion, neuroinflammation, and angiogenesis. In investigating research on angiogenesis and autism, we found a 2016 publication concerning autism postmortem brains which identified persistent splitting angiogenesis, as opposed to sprouting angiogenesis, in some regions of all donated autism brains and in none of the age-matched control brains. With this finding and the established effects of hyperoxia on angiogenesis and inflammation, we have developed a hypothesis that potentially accounts for regional cerebral hypoperfusion; delayed, progressive onset of ASD over the early years of life; increased perfusion in hypoperfused regions of the brain following hyperoxic therapy; the broad range of oxygen partial pressures that seem to have impact; permanent reduction in the symptoms of autism resulting from MBO2; and perhaps, a time-efficient and cost-effective method of treating autism that will help upgrade the long-term prognosis for affected individuals.
Normobaric; Hyperbaric; Atmospheric; Microbaric; Hyperoxia; Hypoxia; Neuroinflammation; Hypoperfusion; Angiogenesis; Sprouting; Splitting; Intussusceptive
Present Treatment of Autism Spectrum Disorders
Despite the rapid growth in the prevalence of autism spectrum disorders (ASD) and its huge, expanding financial impact, a generally accepted intervention or group of interventions which consistently and comprehensively address the spectrum of needs of most individuals with autism in a cost-effective and timeefficient manner has not yet been identified or developed [1-9]. This includes behavioral/psychosocial interventions such as those based on applied behavior analysis (ABA) which, since the 1970s, have become the most recognized and common forms of case management [4,10-17]. In reality, however, because of great individual heterogeneity in factors like the genes and neurocognitive mechanisms involved in individual cases of ASD and lack of comparative, randomized, controlled studies, no intervention pathway comes with any assurance that it will be successful for any particular case [4,5,9].
When the interventions tried for ASD do not achieve the desired objectives, parents ultimately may be faced with a decision as to whether or not to employ pharmaceutical agents such as psychotropic, antipsychotic, antidepressant, and/or ADHD drugs to suppress specific associated behaviors in a person with autism such as irritability, aggression, self-injury, anxiety, hyperactivity, impulsivity, inattention, and insomnia [15,18]. Many opt for this “out” as the child ages, even though there are a great number of undesirable side-effects to psychotropic and antidepressant agents including anxiety, blurred vision, dizziness, fatigue, increased appetite and weight gain, insomnia, nausea, parkinsonism, restlessness, and weakness [19-21]. An extensive review of drug use (i.e., one or more such pharmaceuticals) considered 47 studies published in either English or German in peer-reviewed journals collected from 1976 to 2012 [22]. Data from more than 300,000 individuals with autism were included. Table 1 gives the median drug use for different types of agents ranging across all ages, children, adolescents, and adults.
| Drug Type | All with ASD | Children | Adolescents | Adults |
|---|---|---|---|---|
| Psychotropic | 45.7% | 41.9% | 42.5% | 61.5% |
| Antipsychotic | 18.1% | 16.6% | 16.8% | 42.8% |
| Antidepressant | 17.2% | 12.2% | 21.7% | 35.7% |
| ADHD/Stimulant | 16.6% | 19.0% | 13.9% | 11.2% |
Table 1. Nature and median prevalence (%) across studies of drug use by individuals with autism in different age ranges (i.e., all ages; children <12 years; adolescents 12-17 years; adults >17 years).
One important aspect of present non-pharmaceutical interventions for ASD is that even some of those that have shown promise seem to be less effective as the patients being treated become older or have not been investigated for use in older patients [4,10,23-25]. Indeed, we have found that the age qualifier, “young” generally referring to children five years of age or less, is in the titles of many scientific publications concerning the efficacy of ABA-based therapies for autism [23,26-34].
Autism is a Biological Disorder
Research has clearly established autism as a multi-system disorder that impacts not only the brain, but the immune system, the gastrointestinal tract, and other organ systems [35]. The existence of underlying morphological and physiological abnormalities in the autism brain was recognized in the 1960s [36,37]. Subsequent research has now involved many regions of the brain and many technical approaches. These include blood and urine analyses; pneumoencephalography (PEG); electroencephalography (EEG); scans with a variety of imaging techniques and tracer substances; histological and histochemical analyses of postmortem brains and brain biopsies. As a consequence, it is clear that there is a wide range of brain pathology in autism with multiple regions involved.
In view of the extensive pathological findings in autism brains, it has been stated and reiterated that ASD has a biological rather than a psychological basis [12,38-46]. Despite the volume of such data, however, it has not been possible to construct a coherent anatomical and/or pathophysiological basis for autism [5,38,46,47]. Attributing this to lack of consistency in research outcomes, Kumar and associates call for even more morphological and physiological research [46].
Microbaric® Oxygen Therapy
As described in our previous publication, with backgrounds in the utilization of oxygen in many aspects of diving and medicine, we wondered if normobaric hyperoxic gas administration might be an effective therapy for autism [48]. This view was based on a number of reports that both routine clinical hyperbaric oxygen therapy (HBO2) and so-called mild hyperbaric oxygen therapy (mHBO2) seemed to provide similar benefits for autism over a wide range of oxygen partial pressures (primarily 0.31 to 1.5 atm) [48-58]. As over half of this range can be administered at normal atmospheric pressure without a whole-body chamber, should it prove effective, then this therapy's convenience, relatively low cost, and safety compared to any form of HBO2 would be improved [59,60]. Also, by eliminating significantly increased pressure, the possibility of conducting longer courses of treatment as well as ethical, prospective, randomized, double-blind controlled studies to verify these results to current scientific standards would be much more likely.
With these possibilities in mind, four independent, geographically separate pilot case studies were conducted on five preteen and teenage boys using a normobaric form of oxygen administration we have called Microbaric® Oxygen Therapy (MBO2) [48]. These treatments were generally conducted by the children's mothers one hour a day for a maximum of five days per week and represent the same initial try-it-and-see evaluation approach for this medical therapy that has been recommended by researchers for untested psychosocial therapies [61,62].
The outcomes of several of these case studies in which treatments extended for up to 18 months and follow-up for over seven years have been dramatic, well beyond what has been reported for any form of hyperbaric oxygen, or for any type of therapy with adolescents the ages of our pilot study subjects (i.e., 10-18 years) that we are aware of [48]. The gains made across the full spectrum of autism symptoms also seem to be permanent (Figure 1) [48]. In view of this, it would seem imperative to conduct randomized, controlled trials to determine conclusively whether or not this therapy offers the promise it would appear to based on our pilot study results [48].
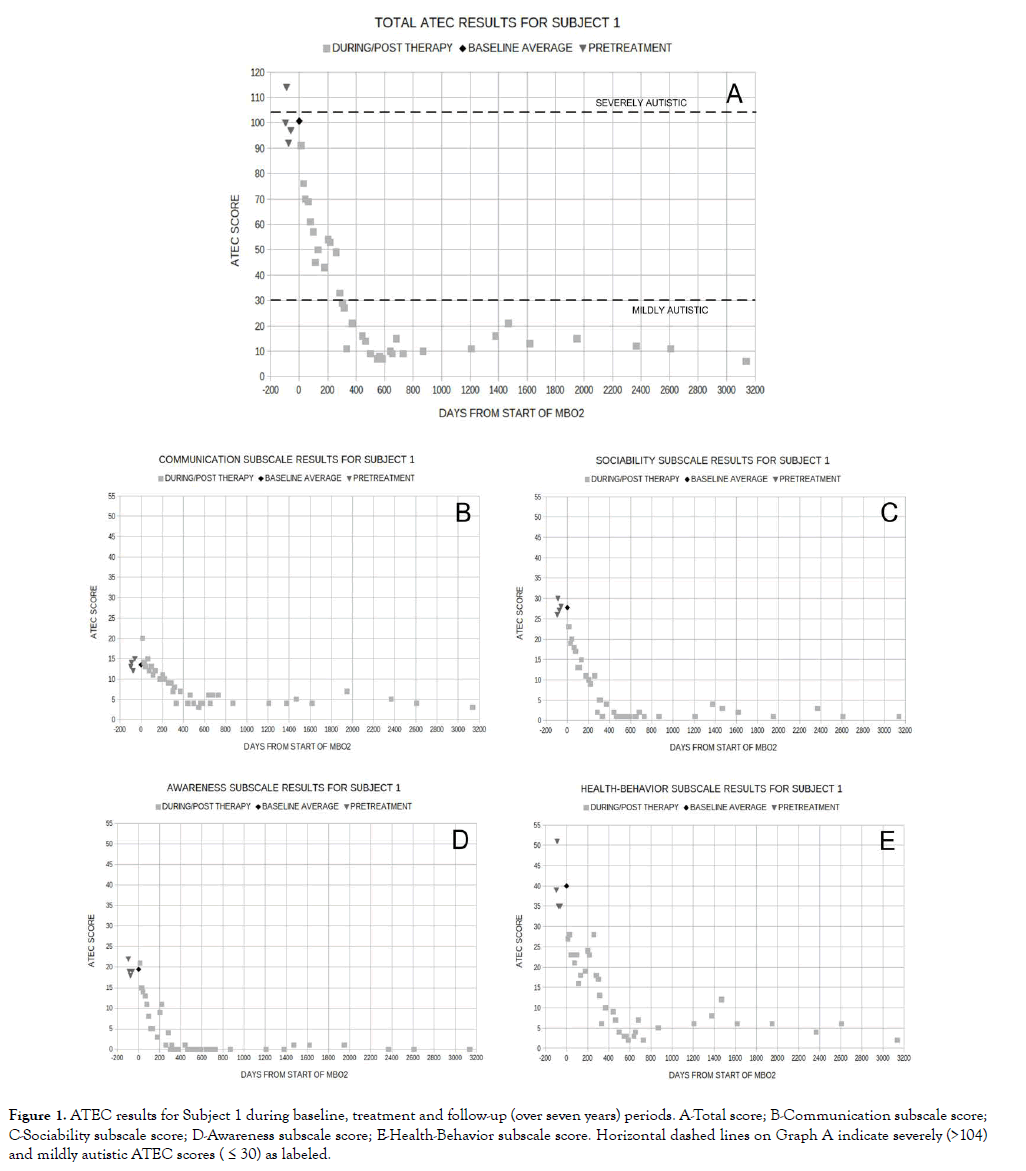
Figure 1. ATEC results for Subject 1 during baseline, treatment and follow-up (over seven years) periods. A-Total score; B-Communication subscale score; C-Sociability subscale score; D-Awareness subscale score; E-Health-Behavior subscale score. Horizontal dashed lines on Graph A indicate severely (>104) and mildly autistic ATEC scores ( ≤ 30) as labeled.
While we believed that a convincing rationale for the efficacy of MBO2 for ASD would not be essential for the conduct of such controlled studies (i.e., the results of our pilot studies would stand on their own merits), we also believe such an explanation will help convince the scientific and support communities concerned with autism that this research is of special importance. In addition, understanding how hyperoxic therapy might benefit ASD could well be a key to both refining this therapy and suggesting fruitful avenues of research to help finally unravel the enigma of autism.
Findings of Interest in the Scientific Literature and Observed in our Pilot Studies
In trying to understand how hyperoxic therapy such as MBO2 benefits those with autism, the factors we believed might be particularly important were:
• Regional cerebral hypoperfusion reported by numerous investigators in association with autism which should produce local hypoxia, hypoglycemia, inflammation and impaired brain function [38,42-45,47,58,63-88] (Table 2).
| Study | YR. | Scan | Tracer/Contrast | Perfusion results | No. Subj. | Subj. Ages (Years) | No. Cont. | Control Ages (Years) | Country of Study |
|---|---|---|---|---|---|---|---|---|---|
| Sherman | 1984 | SPECT | 133Xe | Hypo | 7 | 18-33 | 13 | 20-30 | USA |
| George | 1992 | SPECT | 993TC-HMPAO | Hypo | 4 | 22-34 | 4 | 25-32 | UK/USA/KW |
| Zibovicius | 1992 | SPECT | 133Xe | Normal | 21 | 5-11 (7.5 ± 1.7) | 14 | 8.7 ±1.5 | France |
| Gilberg | 1993 | SPECT | 993TC-HMPAO | Hypo | 31 | 1-22 | 0 | Sweden | |
| Chiron | 1995 | SPECT | 133Xe | Hypo | 18 | 4.5-17.0 (10.9 ± 4.2) | 10 | 4-16 (10.0 ± 3.5) | France |
| Mountz | 1995 | SPECT | 993TC-HMPAO | Hypo | 6 | 9-21 | 7 | 6-20 | USA |
| Zibovicius | 1995 | SPECT | 133Xe | Hypo to Normal | 5 | 2.75-4.33 | 5 | 2.8 ± 0.6 | France |
| Ryu | 1999 | SPECT | 99mTC-ECD | Hypo | 23 | 2.3-7.6 (Mean 4.5) | 0 | SKR | |
| Hashimoto | 2000 | SPECT | 99mTC-ECD | Hypo | 22 | 2-12 | 10 | 2-12 | Japan |
| Ohnishi | 2000 | SPECT | 99mTC-ECD | Hypo | 23 | 2-13 | 26 | 3-12 | Japan |
| Starkstein | 2000 | SPECT | 993TC-HMPAO | Hypo | 30 | (11.1 ± 7.0) | 14 | 11.2 ± 4.3 | AR/USA |
| Zibovicius | 2000 | PET | [15O]H2O | Hypo | 21 | 5-13 (8.4 ± 2.7) | 10 | 5-13 (8.1 ± 2.1) | France |
| Zibovicius | 2000 | PET | [15O]H2O | Hypo | 12 | 7.4±1.7 | Above | Above | France |
| Kaya | 2002 | SPECT | 993TC-HMPAO | Hypo | 18 | 3-11 (6.13 ± 1.99) | 11 | 2-11 (6.5 ± 3.39) | Turkey |
| Wilcox | 2002 | SPECT | 993TC-HMPAO | Hypo | 14 | 3-37 | 14 | 3-37 | USA |
| Ito | 2005 | SPECT | 99mTC-ECD | Hypo | 16 | 9-14 | 5 | 7-15 | Japan |
| Gendry Meresse | 2005 | PET | [15O]H2O | Hypo | 45 | 5.0-11.9 (7.9 ± 2.2) | 0 | France | |
| Burroni | 2007 | SPECT | 99mTC-ECD | Hypo | 11 | 11.2 ± n | 8 | Italy | |
| Degrimenci | 2008 | SPECT | 993TC-HMPAO | Hypo | 10 | 6.9 ± 1.7 | 5 | 6.4 ± 1.4 | Turkey |
| Kinaci, Kinaci | 2008 | SPECT | 993TC-HMPAO | Hypo | 683 | 1-18 | 0 | Turkey | |
| Gupta, Ratnam | 2008 | SPECT | 993TC-HMPAO | Hypo | 10 | 4-8 | 5 | 4-8 | India |
| Yang | 2009 | SPECT | 99mTC-ECD | Hypo | 23 | 7.2 ± 3.0 | 8 | 5.5 ± 2.4 | China |
| Pagani | 2011 | PET | [1-11C] Butanol | Hyper | 13 | 20-48 (31.8 ± 8.6) | 10 | 20-42 (27.8 ± 7.5) | Sweden |
| El-baz | 2012 | MRI | Gadolinium-DPTA | > after HBO2 | 6 | 2-9 | 0 | Egypt | |
| Jann | 2015 | MRI | ASL | Hyper & Hypo | 17 | 13.8 ± 2.0 | 22 | 12.8 ± 3.6 | USA |
Table 2. Studies of brain regional perfusion in autism spectrum disorders.
• Improvements in regional cerebral hypoperfusion following courses of hyperbaric oxygen therapy [54,58,70].
• The long period required to maximize the outcome of MBO2 and HBO2 for neurologic disorders [48,71].
• The apparent permanence of the effects produced by Microbaric® Oxygen Therapy in our case studies [48].
A logical factor to connect these elements would be angiogenesis which is known to be stimulated by hypoxia, promoted by hyperbaric oxygen therapy, and has an integral role in developing the brain's vascular network [89-94]. There seemed to be a fallacy in using straightforward logic to connect these points, however. If regional cerebral hypoperfusion produces local hypoxia, angiogenesis would be expected to occur without the need for hyperoxic therapy, even if such treatment would enhance the response. Further, if angiogenesis occurs, we believed it should improve or correct the hypoxia over time, which based on the considerable research into regional cerebral hypoperfusion in autism over a large span of ages (Table 2), it does not. If this rationale were to have relevance, therefore, we felt there must be a missing element such as a factor inhibiting angiogenesis or causing non-productive angiogenesis. In either case, we expected to find that hyperoxic therapy would change this situation and establish productive angiogenesis in the autism brain.
Identifying a Key Study
In an attempt to explore the possible relationship between hyperoxic therapy and angiogenesis in autism, the terms autism and angiogenesis were entered into the U.S. National Library of Medicine Internet database search engine i.e., PubMed. One of the dozen or so papers that were identified by this search stood out when we read its title, Persistent angiogenesis in the autism brain. An immune cytochemical study of postmortem cortex, brainstem, and cerebellum [95]. Because of the word, persistent, in the title, our immediate thought was that the angiogenesis discussed in this publication must be effectively non-productive. Thus, this angiogenesis would not correct the local hypoxia, which would, therefore, continue to stimulate ongoing or persistent angiogenesis.
The type of angiogenesis found by Azmitia and associates was splitting (aka, intussusceptive (i.e., from within) or non-sprouting) [95]. In comparison to sprouting angiogenesis, first described in the late eighteenth century by John Hunter and which generally comes to mind when the term, angiogenesis, is used, splitting angiogenesis was only recognized in the mid-1980’s [96,97]. In sprouting angiogenesis, entirely new capillaries are formed when endothelial cells bud, migrate, and proliferate [98,99]. This makes it possible for developing capillary networks to traverse avascular and hypoperfused tissue such as may exist in traumatic wounds [100,101]. Splitting angiogenesis, on the other hand, divides existing capillaries to form a denser network within a highly localized, already perfused area [102,103]. Thus, splitting angiogenesis does not create new vessels capable of traversing avascular tissue, and consequently hypoperfused zones could be created and persist in growing tissue that was limited to the splitting type of angiogenesis. The report by Azmitia and associates, therefore, seemed to provide the critical element we had hypothesized might exist [95].
Based on these facts from the scientific literature as well as plausible assumptions including an alternative interpretation as to why Azmitia and associates found splitting angiogenesis in all of their autism brains, we have developed a hypothesis that accounts for regional cerebral hypoperfusion; delayed, progressive onset of autism spectrum disorders over the first several years of life; increased perfusion in hypoperfused regions of the brain following a course of hyperoxic therapy; the broad range of oxygen partial pressures that seem to have impact on the symptoms of autism, including oxygen pressures administered to air-breathing control subjects at increased pressures in whole-body chambers; permanent reduction in the symptoms of autism resulting from hyperoxic therapy; and perhaps, a time-efficient and cost-effective method of treating autism that will significantly improve the long-term prognosis for many individuals affected by ASD [95]. The evolution of this hypothesis and how we believe it may address these various factors is the primary focus of this publication.
Regional Brain Hypoperfusion in Autism
Since the mid-1980s, functional neuroimaging showing tissue blood flow and metabolic activity levels in the autism brain has been conducted with single-photon emission computed tomography (SPECT) and positron emission tomography (PET) scans. Tracers for SPECT scans measuring regional brain perfusion have included technetium-99m (99mTC) in both ethyl cysteinate dimer (ECD) and hexamethylpropyleneamine oxime (HMPAO) complexes, and 133Xenon (133Xe). Tracers for PET scans measuring regional brain perfusion have included [15O] labeled water (i.e., [15O] H2O) and [11C] butanol. More recently, magnetic resonance imaging (MRI) scans have been utilized with gadolinium-DTPA as a contrast agent and with arterial spin labelling (ASL) which does not require the administration of an exogenous contrast agent [58,88,104,105].
With these various approaches, a number of studies from around the world involving over one thousand subjects have generally shown that individuals with autism have significant hypoperfusion in one or more regions of their brains. As with other studies of physiological and morphological abnormalities in autism, however, these results are not entirely consistent (Table 2). This may in some part be due to differences in the regions of the brains studied; the degree to which individuals are affected by autism; and the assessment techniques, methods of subject management, and control methods for the regional brain perfusion assessments.
Re the latter point, radioactive tracer substances were used for the SPECT and PET scans, and a toxic contrast substance (i.e., Gadolinium) was used for one of the MRI assessments. Thus, there have been ethical challenges as to how to compare measurements in subjects with autism to those in non-autistic, age-matched control subjects. One solution has been to compare the autism scans to scans made with the same method for other purposes in individuals who were found to have normal brains [47,74-76,80,81,84,86]. Another solution has been to do control measurements on subjects with non-autistic retardation and/or seizure disorders [43,77-79,82]. Yet another solution has been to do control measurements in normal subjects [38,44,45,72,75,87,88].
In other studies, approaches that did not involve taking scans in control subjects have been utilized. One of these was to use multiple, experienced, blinded nuclear medicine radiologists to read the scans and determine outcomes based on their consensus judgments [42,73,85]. Another was to use statistical evaluation methods (i.e., Covariance analysis) [83]. Lastly, El-baz and associates normalized their data to white matter perfusion measurements and compared regional brain perfusion outcomes in the same subjects before and after courses of hyperbaric oxygen therapy [58].
With regard to the regional cerebral perfusion outcomes for the twenty-four studies listed in Table 2, only four had outcomes with some degree of inconsistency from the other studies [47,76,87,88]. The other twenty studies using a variety of scan techniques found hypoperfusion in one of more regions of the brains of subjects diagnosed with developmental disabilities on the autism spectrum [38,42-45,58,72-86]. These subjects ranged in age from one to thirty-seven years. As this represents the predominant finding, by far, of the relevant research we have identified, this outcome is used in our analyses below.
The twenty studies referenced above included 1,033 subjects on the autism spectrum compared to 150 age-matched control subjects. Of the subjects with autism, individual data are presented for 810 subjects, of which 799 (i.e., approximately 99%) were found to have at least one cerebral region with significant hypoperfusion [42,45,58,72,73,75,79,81,85]. In the other studies involving 223 subjects, the data were analyzed statistically in groups which were found to have significant regional cerebral hypoperfusion [38,43,44,74,77,78,80,82-84,86].
Some of the investigators referenced above have suggested that the hypoperfusion they found is an important factor in the developmental disabilities of autism and two other studies have shown strong correlations between the extent of specific regional hypoperfusion and the degree of specific symptoms of autism [38,43,44,45,77,81-83]. Additionally, Yang and associates assessed regional cerebral perfusion in separate groups of children diagnosed with autism and Asperger's syndrome, respectively [86]. While both groups had hypoperfusion in some regions compared to controls, the patients with autism had lower perfusion values as a group than the usually higher functioning Asperger's patients. Lastly, two studies, one a subset of 108 subjects from among the 683 reported by Kinaci and Kinaci and included in Table 2, have reported regional cerebral hypoperfusion to be significantly improved and autism symptoms decreased following courses of hyperbaric oxygen therapy [54,58,85].
Though no cause-and-effect relationship has been conclusively established between the improvements in regional cerebral perfusion and the symptoms of autism cited above, the circumstantial evidence for such a relationship would seem strong. The brain is metabolically one of the most active of all organs in the body. At about 2% of total body weight, it accounts for approximately 20% of the resting total body oxygen consumption [106,107]. It is of no surprise, therefore, that brain function and repair are critically dependent on adequate supplies of both oxygen and glucose [63- 69]. A large body of evidence suggests that the partial pressure of oxygen (PPO2) in brain tissue is physiologically maintained within a narrow range in accordance with region-specific activity under normal circumstances. Such auto regulation of cerebral circulation, neurovascular coupling, ensures that regions of the brain receive the necessary supply of oxygen despite variations in perfusion pressure and inspired oxygen levels [94,108-114].
With regions of the autism brain apparently chronically hypoperfused, it seems unlikely that the circulation in those regions would be capable of an autoregulatory response adequate to correct hypoxia and maintain normal brain function (Table 2). The persistence of the stimulation of angiogenesis in brain regions identified by Azmitia and associates effectively confirms this [95]. Thus, this finding together with the hypoperfusion found by many investigators in the autism brain suggests an ongoing cycle of hypoperfusion, hypoxia, and effectively nonproductive angiogenesis leading to pathological brain function (Table 2). It is interesting to note that chronic brain hypoperfusion producing cerebral hypoxia and glucose hypometabolism has been implicated in a number of CNS conditions including neurodegeneration, cognitive impairment, and dementia [67-69,115].
Based on reported improvements in symptoms of autism following courses of hyperbaric oxygen therapy and Microbaric® Oxygen Therapy, and concomitant increases in perfusion in some regions of the autism brain, we have concluded that periodic hyperoxic therapy has a positive effect on cerebral blood flow in compromised regions of the autism brain [48-58]. This improves local brain oxygenation, metabolism, and function, reducing related symptoms of ASD. The challenge produced by this conclusion that hyperoxic therapy improves regional cerebral blood flow in compromised regions is determining how these treatments over a relatively wide range of oxygen tensions have such influence. One focus of attention in this regard has been angiogenesis.
Angiogenesis
Angiogenesis is the process that establishes the capillary beds in new tissue growth, tissue regeneration, and remodeling of the microvascular network to create the adult circulatory system [102]. Angiogenesis is known to occur by two distinct mechanisms, sprouting and splitting (aka, intussusceptive or non-sprouting). Both processes are triggered by the up-regulation of angiogenic growth factors such as VEGF (vascular endothelial growth factor) expressed by hypoxic tissues [90,116-120]. Angiogenesis occurs throughout life in health and disease, beginning in utero and continuing through old age [102].
Because splitting angiogenesis does not depend on immediate endothelial cell proliferation or migration, or on basement membrane degradation and invasion, it is a rapid mechanism of remodeling or repair, as well as an energy-efficient one [121]. It plays a prominent role in vascular development in embryos where growth is fast and resources are limited [122]. Also, because of the rapidity with which it creates new capillaries and increases local oxygen levels, splitting angiogenesis may have an important, perhaps evolutionary role in the body's response to acute trauma. It has been looked for and found in a great many organs including the eye, sub-mandibular salivary gland, heart, liver, stomach, small and large intestines, trachea, kidney, uterus, and ovary [123].
Splitting Angiogenesis and Autism Spectrum Disorders
As already described, several case studies treating ASD with Microbaric® Oxygen Therapy produced significant improvements across the full range of symptoms of autism, and these outcomes seem permanent [48]. In addition, two studies treating a total of 114 cases of ASD with hyperbaric oxygen therapy have not only produced improvement in the symptoms of autism but also significant concomitant increases in the perfusion of hypoperfused regions of the brain [54,58,95]. With the research of Azmitia and associates establishing the existence of splitting angiogenesis in some regions of all the postmortem autism brains they examined, the possibility that regional cerebral hypoperfusion in autism and the beneficial effects of hyperoxic therapy (i.e., MBO2, HBO2, and mHBO2) are related through splitting angiogenesis is considered here.
In their research, the first cellular study of blood vessels in the autism brain, Azmitia and associates used immunocytochemical methods to examine the postmortem brains of ten individuals diagnosed with autism [95]. These donors ranged in age from 2.8 years to 28 years with a mean age at death of 14.5 years. These autism brains were compared to postmortem brains from ten donors, none of whom had any known psychiatric diagnoses [95]. These controls ranged in age from 1.8 years to 32 years with a mean age at death of 15.1 years.
The points of comparison for the autism and control brains in the Azmitia study included four non-angiogenic markers and two angiogenic markers, nestin and CD34. These angiogenic markers are localized to pericytes and endothelial cells, respectively, and indicate ongoing angiogenesis [124,125]. There were no differences between the autism and control brains with respect to the nonangiogenic markers, but distinct differences with respect to both angiogenic markers.
Azmitia and associates observed that the "angiogenic markers (nestin and CD34) were found to be highly increased in ASD and almost nonexistent in controls" [95]. Nestin-positive pericytes covered blood vessels in all of the autism brains from 2.8 to 28 years, but only in the control brains from the two youngest donors (i.e., 1.8 and 2.1 years) who would still have been in a period of rapid brain growth and vascular system development (Figure 2) [126,127]. From 8.5 to 32 years, the brains of the control subjects were devoid of nestin-positive pericytes [95]. CD34-positive endothelial cells were associated with pre-capillary arterioles and capillaries in all of the autism brains, but in only one of the control brains, that of an 8.5 year old boy who died of respiratory distress [95]. In this latter case, the severe respiratory distress quite likely led to brain hypoxia and consequent increases in VEGF levels and brain angiogenesis [128]. Consequently, all of the autism brains had both angiogenic markers associated with blood vessels and a labeling profile indicative of splitting rather than sprouting angiogenesis [95]. Only three of the control brains had a single angiogenic marker, however, and none had the full labeling profile of splitting angiogenesis [95].
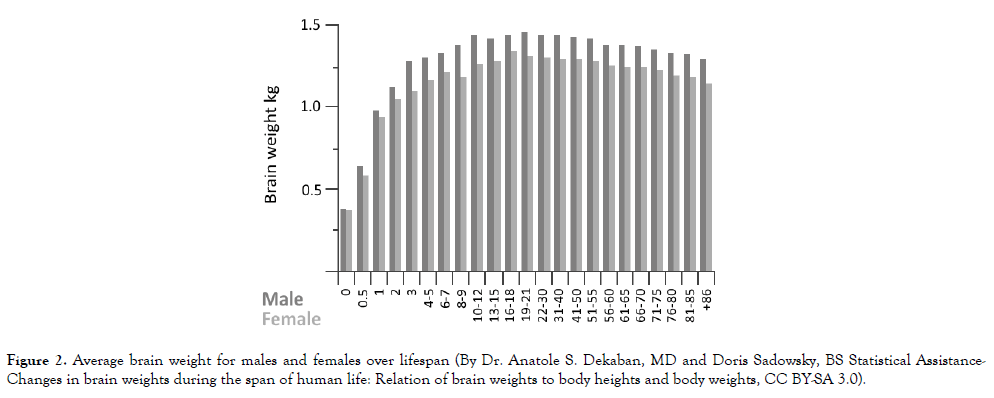
Figure 2. Average brain weight for males and females over lifespan (By Dr. Anatole S. Dekaban, MD and Doris Sadowsky, BS Statistical Assistance-Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights, CC BY-SA 3.0).
As previously stated, our immediate reaction to the word, persistent, in the title of the Azmitia publication was that the angiogenesis being referred to in the autism brains was not productive, and this expectation would seem to have been met [95]. Instead of development of an expanded vascular network such as would be required to adequately support a rapidly growing brain, splitting angiogenesis produces a denser network of capillaries in areas where they already exist [102,103].
The explanation given by Azmitia and associates for the existence of splitting angiogenesis in the autism brains is history of seizures and/or the taking of serotonin drugs (eg: drugs with psychotropic effects such as antidepressants which are frequently prescribed for difficult-to-control cases of autism) [18,22,95]. Upon closer inspection, the possibility that all ten of the autism brains should have splitting angiogenesis for such reasons seems unlikely, however.
On the basis of simple probability using published prevalence of seizures and the taking of serotonin drugs in autism (i.e., specific serotonin reuptake inhibitors which act as antidepressants), the likelihood for a single individual with autism to have either one or both of these conditions would be more likely than not [i.e., approximately 65 out of 100 (If 46 out of 100 individuals with autism, as the worst-case average, have seizures and 36 (i.e., 35.7%) out of 100, as a worst case average, take SSRIs, then approximately 65 out of 100 will have either seizures and/or take serotonin drugs (i.e., 0.46+(1-0.46)*0.357=0.653)) worst case]. On this same basis, however, an entire group of ten individuals with autism all having a history of either seizure and/or taking serotonin drugs would be unlikely, expected to occur in less than 2 of 100 cases (0.65310=0.01405) on average. Thus, even if all of those with autism having a history of seizures and/or taking serotonin drugs would be found to have splitting angiogenesis in one or more regions of their brains, it would be highly unlikely that any random group of 10 autism brains would all have splitting angiogenesis as found by Azmitia and associates [95].
In actual fact, the medical profiles of the individuals with autism donating brains for the Azmitia study indicated that only seven of the ten had a history of seizures and/or of taking serotonin drugs. The other three, among the five youngest donors including the 2.8 year-old, had no history of either seizures or taking serotonin drugs. Thus, their own data do not fully support the explanation given by Azmitia and associates for the occurrence of splitting angiogenesis in all of their autism brains [95].
In addition, should splitting angiogenesis result from just seizures associated with autism or drugs being taken because of autism, then such angiogenesis and the regional cerebral hypoperfusion commonly found in autism brains could not be related. The logic for this conclusion is based on two points.
First, regional cerebral hypoperfusion has been found to occur in a substantially larger percentage of the autism population (i.e., approximately 99% from the derivation in the section, Regional Brain Hypoperfusion in Autism, earlier in this paper) than that which is likely to have a history of seizures and/or the taking of serotonin drugs (i.e., a maximum of about 65% as noted immediately above).
Second, Gillberg and associates found no practical differences in regional cerebral hypoperfusion between 16 autism patients with seizure histories and 15 without seizure histories [73]. This suggests that either seizures are not a primary cause of splitting angiogenesis in those with autism, or if they are, then in order to match the finding of Azmitia and associates, every case of autism without a history of seizures must have a history of taking serotonin drugs [95]. As this latter fact is neither supported by reports on serotonin drug prevalence in the autism literature nor by the profiles of the ASD donors for the Azmitia study, some other explanation for a relationship between splitting angiogenesis and regional cerebral hypoperfusion, or no relationship at all, must be considered (Table 1) [22,95].
As a consequence, while all the “pieces” necessary to tentatively solve a significant element of the “autism puzzle,” appeared to be available, with the cause of splitting angiogenesis as suggested by Azmitia and associates, these "pieces" could not be assembled into a coherent whole. By adopting an alternative explanation for the presence of splitting angiogenesis in 99% of autism brains, however, this situation was reversed. For this, we made the following assumptions [95].
In 10 studies including 810 subjects with autism ranging in age from 1 year to 37 years whose outcomes were given individually, approximately 99% were found to have hypoperfusion in some regions of their brains [42,45,58,72,73,75,79,81,85]. In 10 other studies including 233 subjects with autism ranging in age from 2 years to 33 years whose outcomes were determined statistically for the groups, it was found that the autistic subjects had significantly lower perfusion rates in one or more areas of the brain [38,43,44,74,77,78,80,82,83-84,86]. Taking this prevalence and these age ranges into account, it is assumed that hypoperfusion in one or more regions of the autism brain is generally a life-long condition associated with autism, just as autism, itself, is a lifelong affliction.
The donors of the autism brains for the Azmitia study also spanned a relatively broad age range (i.e., 2.8 to 28 years), and contrary to the authors' speculation that such splitting angiogenesis results from some specific autism-related history (i.e., seizures and/or the taking of serotonin drugs) which those with autism may or may not have, every one of the brains in this group of subjects had splitting angiogenesis despite the fact that some did not have the requisite medical history specified by Azmitia and associates [95]. In view of these facts, it certainly seems plausible that splitting angiogenesis in one or more regions of the autism brain is a common, lifelong condition, and we make this assumption in developing the hypothesis presented here.
Splitting and sprouting angiogenesis are complementary processes in the formation of normal, fully functional vasculature with sprouting angiogenesis linking the primary supply and drainage systems formed by vasculogenesis and splitting angiogenesis expanding and finishing the maturation of the capillary beds [119,129-131]. While Azmitia and associates found labeling profiles indicative of ongoing splitting angiogenesis in the postmortem brains of all of their ASD donors, angiogenesis markers were scant in the control brains and none had the full labeling profile of splitting angiogenesis [95]. This suggests that splitting angiogenesis is not common in brains of individuals without autism, thus making the evidence for splitting angiogenesis in all of the autism brains particularly notable. Since a combination of sprouting and splitting angiogenesis in response to hypoxia in hypoperfused regions of the autism brain would be expected to correct the hypoxia and remove the angiogenic stimulus, it seems logical to conclude that in hypoxic regions found to have persistent splitting angiogenesis in autism brains, sprouting angiogenesis has been impaired or impeded. If sprouting angiogenesis existed in these regions, it should correct the hypoxia stimulating angiogenesis in the first place. Such a situation in which splitting angiogenesis predominates over sprouting angiogenesis is not without precedent in other pathology. In Krabbe disease, for instance, alterations of the functional angioarchitecture of the brain cortex were found in a murine model, the twitcher mouse, and would seem to be produced by impaired sprouting angiogenesis being replaced by splitting angiogenesis [132.133].
As the regions of the autism brains Azmitia and associates found to have persistent splitting angiogenesis and the regions of the autism brains many investigators have found to have significant hypoperfusion overlap, a logical relationship between splitting angiogenesis and regions of the brain with low perfusion would seem to be a cause-andeffect one with the lack of sprouting angiogenesis resulting in regional hypoperfusion. This leads to the following explanation for at least some of the important aspects of autism [95].
In-utero or early in life, there is some degree of impairment or failure of sprouting angiogenesis in one or more regions of the brain. In these regions, splitting angiogenesis may serve as an escape mechanism to try to overcome the defective sprouting angiogenic responses as Giacomini and associates have suggested for Krabbe disease, and Dimova and Djonov have suggested for during and after irradiation and anti-VEGF therapy [133,134]. As this is at an age when the brain is growing rapidly, perfusion and oxygenation become progressively more inadequate in those regions, and the brain ceases to function normally as the child develops. He thus falls behind normal children. Unless the regional brain perfusion is corrected, which does not seem likely to be a common event in autism, the resulting developmental disabilities will increase through the period of brain growth, or at least rapid brain growth, and last for the individual’s lifetime.
This sequence would account for individuals with autism appearing normal or essentially normal at birth and then developing progressively greater symptoms as they grow older [135- 140]. It would also account for the consistent finding of splitting angiogenesis in some regions of the autism brain by Azmitia and associates and in the great prevalence of regional cerebral hypoperfusion in the autism brain found by many investigators [Table 2] [95]. Following this line of logic, the next issue is how hyperoxic therapy improves this situation.
Working Hypothesis of How MBO2 Improves the Symptoms of Autism
Following on from the description of autism given above, it is hypothesized that serial MBO2 causes the nature of angiogenesis in the hypoperfused, and therefore hypoxic regions of the brain to change from splitting to sprouting. Over time, sprouting angiogenesis expands capillary networks throughout the hypoperfused regions of the brain making them better able to oxygenate and provide metabolites to those areas. With improved metabolic capacity and, therefore, brain function, development that has stalled or even regressed is restarted so that the symptoms of autism diminish. As envisioned here, the change in angiogenesis from splitting to sprouting is independent of the reason splitting angiogenesis predominates in regions of the autism brain to begin with. In other words, this hypothetical rationale for the efficacy of MBO2 for autism is independent of whether or not autism is fundamentally a genetic disorder, an environmental disorder, some combination of these two, or something entirely different.
As illustrated by our case study Subject 1 (Figure 1) and Subject 2 (Figure 3), the outcomes of MBO2 have been progressive and taken some time (on the order of 1½ years) for significantly disabled teenagers to plateau with the only remaining symptoms of autism classified as mild [48]. This finding is similar to that for the success of HBO2 in treating stroke which has been shown to be directly related to the duration of treatment [71].
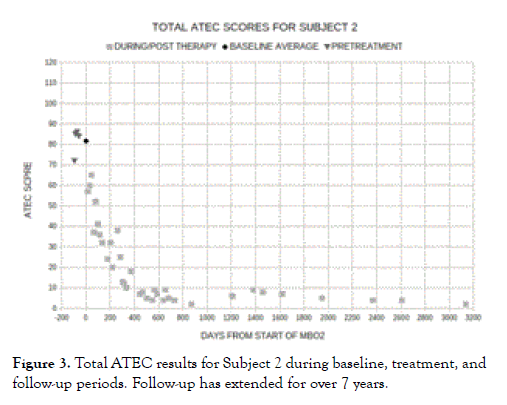
Figure 3. Total ATEC results for Subject 2 during baseline, treatment, and follow-up periods. Follow-up has extended for over 7 years.
As further illustrated by our case study Subject 4 (Figure 4), the longterm steady improvement in the symptoms of ASD stopped when treatment was terminated. Within the context of the hypothesis presented here, this would seem to indicate that the promotion of sprouting angiogenesis in place of splitting angiogenesis by hyperoxic therapy only occurs over the general time frame that the therapy is being administered. Thus, when Subject 4's family moved after approximately a year of MBO2 and terminated that therapy, the steady improvement in Subject 4's symptoms of autism halted. In keeping with the hypothesis that such improvement is through permanent expansion of capillary networks leading to enhanced delivery of oxygen and metabolites to previously hypoperfused regions of the brain, however, the reduction in symptoms of autism did not regress after the treatment was stopped.
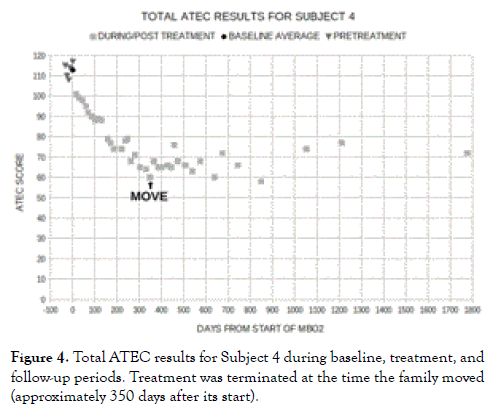
Figure 4. Total ATEC results for Subject 4 during baseline, treatment, and follow-up periods. Treatment was terminated at the time the family moved (approximately 350 days after its start).
For hyperoxic therapy to have such impact in the autism brain, it would seem essential that oxygen have some role in the regulation of angiogenesis. Review of the scientific literature confirms that angiogenesis is controlled by, among other factors, variations in tissue oxygen tensions [141].
As prominent roles of the circulatory system are to deliver oxygen and metabolic substrates to tissues, it should not be surprising that regulation of the initial and ongoing development of the circulatory system throughout life involves oxygen and metabolic intermediates as triggers for complex, reactive control processes involving sensing systems, signaling molecules and pathways, and substances such as angiogenic cytokines (eg: VEGF) which up-regulate or downregulate genes to effect or alter angiogenesis [141]. In these control processes, oxygen levels from hypoxic to hyperoxic are detected and reacted to independently from metabolic intermediates (eg: glucose) [141].
One of the factors mentioned above, VEGF, has been identified as a prominent element in the control of angiogenesis [90,117- 120,142]. It is a major positive regulator of sprouting angiogenesis; a trigger for angiogenesis in response to hypoxia and/or hypoglycemia through gene transcription in response to hypoxiainducible factor-1 (HIF-1); and a trigger for vascular pruning in response to hyperoxia [144-148].
Oxygen is also involved in the processes controlling angiogenesis as a signaling element. Though traditionally viewed as byproducts of oxygen metabolism toxic to biomolecules, reactive oxygen species (ROS) (eg: Peroxides, superoxide, hydroxyl radical, singlet oxygen) are now recognized as important modulators of biologic processes and pathologies including angiogenesis and other aspects of cardiovascular system development and regulation [120,149,150]. ROS involved in signaling are produced at low levels in endothelial cells by an NADPH (Nicotinamide Adenine Dinucleotide Phosphate) oxidase activated by angiogenic factors (eg: VEGF, angiopoietin) and in mitochondria by the electron transport chain in inverse relationship to local tissue oxygen tensions [120,146,151- 153].
In these capacities, it has been found that ROS interact with the Notch system, an intercellular signaling pathway related primarily to sprouting angiogenesis [120,151,152,154]. The inhibition of Notch has been shown to produce splitting angiogenesis [134,154,155]. ROS produced by the mitochondria are required for the normal induction of HIF, the master regulator of genes that are activated by low levels of oxygen and play roles in acute and chronic adaptation to oxygen deficiency including angiogenesis [147-149].
In view of such varied aspects of oxygen involvement in the control of angiogenesis, a hypothesis based on hyperoxic therapy changing angiogenesis from splitting to sprouting in hypoperfused regions of the autism brain certainly seems plausible. A specific mechanism for this effect cannot be delineated at this point, however. While the molecular mechanisms relating to sprouting angiogenesis have been well investigated, those regulating splitting angiogenesis remain unclear [119,134,155,156]. It is known, however, that angiogenesis is a sophisticated multistep process tightly regulated by “on-off switch signals” triggered by changes in the relative balance of proangiogenic factors such as VEGF and angiogenic inhibitors such as thrombospondin-1 [157]. Fine temporospatial tuning between sprouting and splitting angiogenesis is regulated in part by the interplay between VEGF and basic fibroblast growth factor (bFGF) [158]. Thus, it seems logical and in keeping with evolutionary principles that the ready availability of oxygen, which supports energy-dependent processes, would favor sprouting angiogenesis while hypoxia, which would not support energydependent processes, would favor splitting angiogenesis.
These factors suggest that the effect of hyperoxic conditions could simply be to "flip a switch" to turn on sprouting angiogenesis instead of splitting angiogenesis. If this were the case, it seems likely that a wide range of higher-than-normal oxygen tensions could have such an effect as has been observed with the hyperoxic treatments of autism from mHBO2 to MBO2 to HBO2 (primarily from 0.312 atm for mHBO2 to 1.50 atm for HBO2). That the low end of this PO2 range has effects similar to those of the high end in the brain, though perhaps over a smaller volume of tissue, is in keeping with a point made by Christian J. Lambertsen, a pioneer in research on oxygen effects and tolerance [159]. He stated that "the physiological effects of oxygen at increased tension are most prominent in those tissues and organs or in the chemical processes which are also most subject to influence by abnormal PO2". Certainly, the brain qualifies as such an organ.
Neuroinflammation and an Alternative Mechanism for the Benefits of MBO2 for ASD
The brain’s substantial energy requirements and limited capacity to store glucose each factor into the process resulting in hypoxic tissue damage and abnormal brain function when the vascular supply is compromised [94]. The induction of inflammation by hypoxia is now generally accepted, and studies of hypoxia signaling pathways have shown that hypoxia and inflammation are linked at the molecular, cellular, and clinical levels [65,67,68]. Hypoxia has an active influence over the environment of the inflamed tissue, particularly through the regulation of oxygen-dependent gene expression [160].
While the specific links between immune system dysfunction, neuronal dysfunction, and developmental disorders are still unresolved, several lines of evidence highlight the involvement of altered immune responses in the pathogenesis of ASD [35]. Vargas and colleagues used immunocytochemistry, cytokine protein arrays, and enzyme-linked immunosorbent assays to study brain tissue and cerebrospinal fluid (CSF) from autistic patients [161]. They demonstrated an active neuro-inflammatory process in the cerebral cortex, white matter, and cerebellum of patients with autism and an increased presence of pro-inflammatory cytokines in the CSF [161,162]. Subsequent studies using post-mortem brain tissue have further demonstrated CNS inflammation in persons diagnosed with ASD and consistently found neuroinflammation in postmortem autism brains from individuals over a broad age range (i.e., 5-44 years) [48,163]. This suggests that neuro-inflammation could be a life-long symptom of autism.
As hypoxia has been shown to produce inflammation, one possible cause of the neuroinflammation associated with autism could be hypoxia resulting from the hypoperfusion found to exist in one or more regions of the autism brain by many investigators (Table 2) [65,67,68,160]. Such a relationship has been suggested in regards to other neurodegenerative and mental disorders [67-69,164]. Miyanohara and colleagues as well as Daulatzai, for instance, have reported that chronic cerebral hypoperfusion (CCH) like that clearly shown to exist in one or more regions of the autism brain by Kinaci and others is a characteristic of neurodegenerative and mental disorders, and that CCH can induce the excessive inflammatory responses that precede neuronal dysfunction and commonly manifest as cognitive impairment [67-69] (Table 2). This sequence of cause-and-effect relationships fits well with the hypothesis implicating splitting angiogenesis as the cause of regional brain hypoperfusion in ASD outlined above.
If the hypothesis presented here for the effects of hyperoxic therapy on autism is correct, therefore, it would seem likely that MBO2 would diminish and could eventually eliminate the neuroinflammation associated with autism. This would be both indirectly by facilitating sprouting angiogenesis and, thus, reducing regional brain hypoxia, and also directly by attenuating inflammation. This latter factor is a recognized attribute of hyperbaric oxygen therapy, and we presume, hyperoxic therapy such as MBO2, which has been demonstrated to have anti-inflammatory effects in a number of conditions and tissues including experimental traumatic brain injury in rat models, ASD in a human study, ulcerative colitis and inflammatory bowel disease in humans and animals, experimental stroke in a rat model, heat stress in diabetic rats, myocardial ischemic reperfusion injury in a rat model, experimental skeletal muscle contusion in a rat model, experimental irradiation of laryngeal tissue in rats, and experimental cord injury in a rat model [52,165-175].
In an ASD study conducted by Rossignol and associates mentioned above, 18 children and adolescents diagnosed as having ASD were divided into two groups [52]. One group was administered HBO2 and the other group mHBO2 [52]. Thus, the oxygen partial pressures of each group were respectively higher and lower than that of MBO2. Relative inflammation in this study was assessed with C-reactive protein (CRP) measured before and after courses of 40 treatments. After these treatments, there was a trend in each group for CRP to be lower than the pretreatment data, but these results were not significantly different (i.e., p was not ≤ 0.05). When the two groups were combined, though, the reduction in CRP post-treatment, and thus, inflammation, was significantly reduced (p=0.021) from the pretreatment level [52].
As well as inflammation resulting from splitting angiogenesis, regional hypoperfusion, and hypoxia, it has also been shown that splitting angiogenesis appears to be a functional adaptation to prolonged inflammation in the colon of the adult mouse and in the brain of the twitcher mouse as a model for human Krabbe disease [133,176]. This opens up the possibility that the relationship between splitting angiogenesis and inflammation in autism might be the reverse of that described in the rationale presented above (i.e., inflammation results from hypoperfusion and hypoxia). If this were the case, though the underlying cause of autism would then likely be different, the ways in which MBO2 impacts positively on ASD would remain essentially the same: Attenuating neuroinflammation and turning on sprouting angiogenesis in affected regions of the brain.
In assessing the likelihood of the two alternative relationships discussed above (i.e., (1) Splitting angiogenesis leading to regional hypoperfusion, hypoxia, and neuroinflammation and (2) Neuroinflammation leading to hypoxia, splitting angiogenesis, and regional hypoperfusion), in relation to our pilot case outcomes, the former alternative would seem the more likely one. With the latter alternative, upon ending MBO2, an underlying issue neuroinflammation could have the potential to start the pathological cycle again, thus producing recurring inflammation, local hypoxia, splitting angiogenesis, and regional hypoperfusion. This, in turn, would cause regression of symptoms of ASD. Over many years of follow-up, however, we have not observed such an outcome in any of our pilot cases [48]. If hypoxia and inflammation were produced by splitting angiogenesis, however, and MBO2 has effectively created adequate regional capillary beds through transient sprouting angiogenesis, then there would seem to be no ready stimulus for reoccurrence of regional brain inflammation and impaired function.
Regardless, regional brain hypoxia produced by some combination of splitting angiogenesis, hypoperfusion, and neuroinflammation would adversely affect the level of oxygen available to support regional tissue function [160,177]. On this basis, the beneficial results achieved during our case studies may, at least in part, result from the reduction in neuroinflammation and the improvement in regional perfusion over the course of Microbaric® Oxygen Therapy for ASD. The view that neuroinflammation is a consequence, rather than the cause of regional brain hypoxia is a better fit with the primary hypothesis described in this presentation, however.
The key points of the hypothetical rationale presented here for the benefits of Microbaric® Oxygen Therapy for autism spectrum disorders are summarized below. This is followed with several points concerning implications of this hypothesis. In all of these points, it is assumed, of course, that the hypothesis is reasonably on target and that primary aspects of MBO2 benefiting ASD continue to be confirmed by appropriate research outcomes.
In and around the time of the brain growth spurt beginning in the third trimester and continuing after birth, sprouting angiogenesis in one or more regions of the brain fails with splitting angiogenesis taking place instead.
The failure of sprouting angiogenesis may be due to some direct factor such as a genetic abnormality or indirectly as the result of local neuroinflammation.
As the brain grows rapidly in early life (Figure 2), vascular expansion in these affected regions fails to keep up, oxygen and metabolite delivery is compromised, neuroinflammation develops, brain function is impaired, and symptoms of autism become apparent. Unless sprouting angiogenesis is restored and inflammation quelled, either spontaneously, and there is no convincing evidence in the scientific literature for this to be common for those with ASD, or as the result of therapy, this situation persists for the life of those afflicted.
So long as it is administered, it seems that MBO2 in appropriate dose either directly causes angiogenesis in affected regions of the brain to change from splitting to sprouting or reduces inflammation which in turn facilitates sprouting angiogenesis. In either case, or perhaps, more likely as a result of the combined effects of MBO2 stimulating sprouting angiogenesis and diminishing inflammation, the vasculature in the deficient regions of the brain expands over time with associated permanent improvement in metabolic capacity, brain function, and symptoms of autism.
With the rationale described here, effectively based on neuroplasticity inducible by hyperoxic therapy over the lifetime of the individual, there would seem to be no age limitations for the beneficial effects of MBO2 on ASD [115]. Though disruption in neuroplasticity may accompany inflammation associated with autism at an early age, both sprouting and splitting angiogenesis are processes that take place throughout life [102,178]. Thus, MBO2 should benefit not only preteens, teenagers, and younger children, but also adults of all ages with autism.
Looking in the other age direction (i.e., to the very young), the implications of this hypothesis, if correct, are profound. If autism could be identified before or around the time that the development of regional vasculature in the brain begins to fall behind organ growth, then in theory, immediate, long-term administration of MBO2 should facilitate essentially normal angiogenesis with correspondingly normal psychosocial development, and consequently, the leading of a normal or near-normal life, despite being afflicted with autism. To put this in other words, if regional brain angiogenesis, and thus, perfusion and metabolic capacity, are maintained at effectively normal levels with MBO2 through the extended period of brain growth, then a child with autism might develop just as a normal child- at least to the extent that regional inflammation and reduced regional metabolic capacity in the brain are responsible for the symptoms of autism. In view of the improvements of Subjects 1 and 2 of our case studies, this would seem to include most if not all of their evident symptoms [48].
With respect to the time and qualifications required for adequate administration of Microbaric® Oxygen Therapy, on the basis of our pilot study results and the hypothesis presented here, the time requirements should not be more than one-hour per day five days a week, and might be less [48]. The days treatments are given on and the time of day treatments are given each day can be made to fit family plans and lifestyle as previously noted. Depending on initial symptoms, a course of MBO2 should range from a minimum of six months to as long as two years. Further research will be necessary to determine the optimal treatment times and frequencies if the results of randomized, prospective, double-blind studies are positive.
The results of our pilot studies showed that these few treatments with hyperoxic gas each week addressed all aspects of the symptoms of autism, unlike current therapies where patient heterogeneity is believed to necessitate an individual approach for the management of each case or groups of similar cases [4,5,7,8,9,25,48,179].
In regards to administration of MBO2, special qualifications, medical or otherwise, are not necessary. All that is necessary for an administrator is being able to supervise the therapy in their home or in special needs facilities, recognize a few contingency situations, and learn how to deal with the simple and logical solutions to such issues. This was readily accomplished by the mothers of the subjects during our case studies [48].
On the bases described here, MBO2 should not only be less costly, but much more convenient for the caregiver, less stressful for the autistic individual being treated for relatively short periods in familiar surroundings, and, therefore, more sustainable over the time frame required to optimize the outcome of a course of treatments.
Finally, by correcting brain pathology associated with autism, MBO2 should not only clear up symptoms of autism on its own, but create patient enthusiasm for other treatments of value and make them more effective and efficient than they have been when administered without such accompanying therapy.
Autism is a biologically based disorder. Consequently, it seems unrealistic to expect that optimal improvements in symptomatology can be achieved without effecting some sort of biological change.
While the hypothetical rationale for the effects of MBO2 on autism presented here would not prevent autism if it is correct, it would produce biological changes that could help correct brain dysfunction and restore capability for those afflicted with autism so that they could be expected to live richer and fuller lives. If administration of MBO2 can be begun early enough, it might be that normal or near-normal development and lives with autism could be a practical reality, even if curing autism is not.
At the very least, improving perfusion and attenuating inflammation in affected regions of the autism brain with MBO2 should improve success and reduce effort in the administration of other therapies capable of further diminishing symptoms of autism.
The costs in money and time for MBO2 would be small in comparison to those same parameters currently being expended to treat and care for individuals with autism using other modalities. Thus, should controlled research prove MBO2 to be as effective as it has seemed in our initial clinical trials, and should a rationale along the lines of the hypothesis proposed here be validated, then MBO2 would have the practical potential to be a safe, simple, and generally effective treatment that could salvage countless lives that would otherwise be lost and to save the world’s economies vast sums at the same time until other research finally determines how to prevent autism. In view of the convenience, cost-effectiveness, time-efficiency, relative safety, and minimal training necessary to conduct MBO2, it is imperative to conduct randomized, controlled studies to determine conclusively whether or not this therapy offers the promise it seems to based on our pilot study results.
Citation: Peterson RE, Allen MW (2020) Hypothesis for How Hyperoxic Therapy May Facilitate Effective Biologic “Correction†of Autism Spectrum Disorders. Autism Open Access 10:250. doi:10.35248/2165-7890.20.10.250
Received: 27-Jan-2020 Accepted: 20-Apr-2020 Published: 27-Apr-2020 , DOI: 10.35248/2165-7890.20.10.250
Copyright: © 2020 Peterson RE, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original work is properly cited.