Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2025)Volume 15, Issue 1
Myocardial fibrosis is a common motif in Heart Failure (HF)-related fatalities. Persistent activation of fibroblasts triggers excessive fibrotic cascades culminating in cardiac fibrosis and dysfunction. However, molecular mechanisms orchestrating fibroblast activation are still obscure and as a result, effective strategies for limiting or reversing fibrosis are lacking. Here, we decrypted the metabolic phenotype of fibrosis-remodelled heart and unlocked bioactive peptide galanin as a metabolic checkpoint in aberrant fibroblast activation dictating fibrotic tissue remodeling. Integrated analysis of RNA-seq revealed a predominant role of galanin in metabolism of cardiac fibroblasts. Functional studies found that galanin calibrates metabolc reprogramming during fibroblast phenotypic switching from naïve to activated phenotype promoting myocardial fibrosis. In a mouse model of HF, chronic treatment with galanin promoted metabolic shift from fatty acid to glucose oxidation, mitochondrial biogenesis and blunted established myocardial fibrosis. Furthermore, we demonstrated that galanin reduced number of CD68-positive macrophages and preserved cardiac function in fibrosis-remodelled hearts. Mechanistically, galanin orchestrated metabolic behavior of aberrantly activated cardiac fibroblasts through the Forkhead box protein O1 (FoxO1) pathway. These findings unravel a previously unknown role of galanin in metabolic rewiring of cardiac fibroblast-tomyofibroblast transition dictating fibrosis resolution and provide a new metabolism-based angle for therapeutic intervention to tackle cardiac fibrosis.
Galanin; Heart failure; Fibrosis; Metabolism; Cardiac remodeling
Fibroblasts represent a multifunctional cell population responsible for the production and turnover of the Extracellular Matrix (ECM) components and fibrosis aggravation. Cardiac fibrosis is a complex progressive disorder dictated by uncontrolled activation of fibroblasts promoting the loss of heart architecture and functional performance. Abundant synthesis of ECM by cardiac fibroblasts remodels the mechanical properties of the myocardium and predispose to Heart Failure (HF) and death. The ultimate step in fibrotic tissue remodeling is the conversion of fibroblasts into aggressive myofibroblastic phenotype characterized by the expression of α-Smooth Muscle Actin (α-SMA). which is incorporated into actin stress fibers and confers a high contractile activity to the cells. Pathologic interrogations have depicted the direct juxtaposition of fibrosis and exaggerated production of Transforming Growth Factor (TGF-β), a key driver of fibrotic disorders. Despite several advances, molecular basis of aberrant fibrogenic behaviors that fibroblasts adapt to TGFβ-triggered ECM remodeling remains obscure [1].
To maintain contractile function, human heart requires perpetually high energy demand, but very limited energy reserves. Consequently, the adequate cell energy metabolism and cardiac performance are tightly interconnected. In healthy heart, there is a distinct and finely tuned balance between the utilization of fatty acids and glucose, the primary substrates for cell ATP provision. In response to physiological and pathological conditions, the heart is adept at shifting its energy substrate preference to adjust the energetic supply. Shift of substrate priority can be beneficial in the short term to ATP calibration, however, it can become deleterious and directs to decline in cardiac function in the long-term. Myocardial substrate preference for energy production is tightly controlled by the transcriptional regulators including Peroxisome Proliferator-Activated Receptor Gamma Coactivator 1 alpha (PGC-1α) and Nuclear Respiratory Factor 1 (NRF1). While the cellular composition of the heart is highly heterogeneous, metabolism-dependent abnormalities in injured heart mainly focus on cardiomyocytes [2]. To date, metabolic distinctions that discriminate naive and agressive behaviors of fibroblasts priming fibrosis resolution are still largely ignored.
The bioactive peptide galanin is a 29 amino or 30 amino acid peptide naturally prevails in the tissues and fluids of mammals. Galanin is broadly dispersed in the central and peripheral nervous systems and the endocrine system. The amino acid sequence of mature galanin is highly conserved between species, reflecting the significance of the endogenous peptide. Galanin acts as modulator, transmitter and trophic factor and is involved in a number of physiological functions including nociception, cognition, sleep regulation, neuroendocrine activities, energy homeostasis and central cardiovascular control. The important roles of galanin have been revealed in pathological conditions such as pain, depression-like behaviour, Alzheimer's disease, epilepsy and addiction. The wide diversity of actions is mediated by three distinct G-protein-coupled receptors subtypes, Galanin Receptor 1 (GalR1), GalR2 and GalR3, which distinguish in their pharmacology, distribution and signaling. Dysregulation of galanin receptors is linked to stroke-related damage, inflammation, diabetes and cancer. However, much of galanin's functional role in cardiovascular system is still undiscovered and characterization of cardiac cell-specific peptide properties remains a challenging task. In this study, we decrypted metabolic reprogramming of fibrosisremodelled hearts in HF phenotype and identify a previously unknown role of galanin in metabolism-dependent transition of fibroblasts from naive to activated phenotype priming fibrosis resolution. Furthermore, we demonstrated that galanin reverses fibroblast profibrotic behaviors and metabolic defects through the FoxO1 signaling pathway.
Animal studies
The investigation conforms to the guidelines from Directive 2010/63/EU of the European Parliament on the protection of animals used for scientific purposes approved by the Institutional Animal Care and Use Committee. Male C57BL/6 mice, 8 weeks old (Janvier Labs., France) were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (8 mg/kg) mixture.
A mouse model of pressure overload was produced by constriction of the ascending aorta for 5 weeks as described previously. Sham-operated mice underwent a similar procedure without ligation of the ascending aorta [2]. Animals were randomly divided into four groups: (i) Sham vehicle (n=6), (ii) Aortic-Banded (AB) vehicle (n=10, (iii) Sham galanin (n=6) and (iv) Aortic-Banded (AB) galanin (n=10). One week after AB surgery, galanin treatment was started (10 μg/kg/day, i.p.) and maintained for 4 weeks.
Echocardiography
Blinded echocardiography was performed as described 25 on isoflurane (5%) anesthetized mice using a Vivid7 imaging system (General Electric Healthcare) equipped with a 14-MHz sectorial probe. Two-dimensional images were recorded in parasternal long and short-axis projections, with guided M-mode recordings at the midventricular level in both views. Left Ventricular (LV) dimensions and wall thickness were measured in at least five beats from each projection and averaged. Fractional Shortening (FS) and Ejection Fraction (EF) were calculated from the twodimensional images.
Histology
Hematoxylin-Eosin ( Hand E) and Masson’s trichrome stainings were performed on 10 μm heart cryosections were done according to standard methods. The extent of cardiac structural changes was quantified using ImageJ software.
Preparation of cardiac fibroblasts, transfection and treatments
Mouse cardiac fibroblasts were isolated and cultured according to the method described previously. Isolated cells were cultured in Dulbecco Modified Eagle Medium-nutrient Mixture (DMEM/ F-12, Invitrogen, France) containing 10% foetal bovine serum (FBS, Gibco, France). Adherent cells were characterized at passage 1 using immunofluorescence microscopy and found to be positive for vimentin but negative for CD31. Fibroblasts up to passage 3 were used in these studies. The experiments with siRNA transfection were performed using LipofectamineRNAiMAX (Life Technologies) according to manufacturer's instructions [3]. Cardiac fibroblasts were subjected to 10 ng/ml TGFβ for 24 h in presence or absence of 50 nM galanin.
Picrosirius red staining
Following treatment, cardiac fibroblasts were fixed in methanol and incubated in the 0.1% Picrosirius red staining solution (Sigma-Aldrich, France) as per manufacturer's instructions. Picrosirius red was solubilised in 0.1 N sodium hydroxide and the optical density was read at 540 nm (SERLABO Technologies, France).
Reagents and antibodies
Antibodies used in this study are: Anti-FoxO1 (2880) from cell signaling and anti-RhoGDI (sc-373723) from SantaCruz Biotechnology; anti-galanin (ab-175487) from Abcam; and anti- CD68 (MCA1957GA) from Bio-Rad (formerly AbDSerotec) [4].
Galanin was purchased from GeneCust and was referred to as Gal throughout this study. All other chemicals were from Sigma- Aldrich unless otherwise stated.
Protein extraction and Western blotting
Proteins from cardiac tissues and cells were extracted using RIPA buffer and quantified using the Bio-Rad Protein Assay (Bio- Rad). Proteins were resolved by SDS-PAGE and western blotting. Immunoreactive bands were detected by chemiluminescence with the Clarity Western ECL Substrate (Bio-Rad) on a ChemiDoc MP Acquisition system (Bio-Rad).
RNA extraction and quantitative Polymerase Chain Reaction (qRT-PCR)
Gene expression was assessed using quantitative polymerase chain reaction. Total RNAs were isolated from cells or mice heart using the RNeasy mini kit (Qiagen). Total RNAs (300 ng) were reverse transcribed using Superscript II reverse transcriptase (Invitrogen) in the presence of a random hexamers. Real-time quantitative PCR was performed as previously described. The expression of target mRNA was normalized to GAPDH mRNA expression [5]. Primers for qRT-PCR used in this study are as detailed in Supplementary Table 1.
RNA-seq library preparation and sequencing
1,250 cells per sample were used for library preparation. All manipulations were made according to the Smart-Seq2 protocol. 27 Libraries were pooled and sequenced using Illumina HiSeq 2500. 8-14M reads per library were obtained.
RNA-seq profiling
The next-generation DNA sequencing workflows require an accurate quantification of the DNA molecules to be sequenced, which assures optimal instrument performance. For this, all DNA libraries were quantified by qPCR. Only DNA with adapters ligated to both ends can be measured, since only these fragments can be amplified to generate material for sequencing. This quantification allowed to determine a DNA library, which is ready to generate clusters to the expected density [6]. Sequencing was then carried out on Paired End 100b reads of Illumina NovaSeq 6000 Image analysis and base calling was performed using Illumina Real Time analysis (3.4.4) with default parameters.
Gene expression analysis in naive (control) and TGFβ-stimulated cardiac fibroblasts was performed using the geneXplain/ BioUML platform and the edgeR tool (R/Bioconductor package) integrated into our pipeline. EdgeR calculated LogFC (the logarithm to the base 2 of the fold change), p-value and adjusted p-value (corrected for multiple testing) for the observed fold change.
Measurements of fatty acid and glucose oxidation
Fatty acid oxidation was measured using [1-14C]-palmitate in heart tissue as previously described. The heart tissue samples were incubated in modified Krebs-Henseleit buffer containing 1.5% fatty acid-free bovine serum albumin, 5 mmol l–1 glucose, 1 mmol l-1 palmitate and 0.5 μCi ml-1 [14C] palmitate (Perkin Elmer, Courtaboeuf, France) for 60 min. After incubation, tissues were homogenized in 800 μl lysis buffer. Complete oxidation was determined by acidifying the incubation buffer with 1 ml of 1 mol l-1 H2SO4 and the 14CO2 was trapped by benzethonium hydroxide (Sigma) placed in a 0.5 ml microtube. After 120 min, the radioactivity was counted (Cytoscint; MP Biomedicals, Strasbourg, France). Similarly, glucose oxidation was assessed by measuring the [14C]-glucose oxidation in cardiac tissue as previously described. Sample incubation for 1 h was performed using a modified Krebs-Henseleit buffer containing 0.2% bovine serum albumin, 20 mM Hepes, 10 mM glucose, 0.8 μCi ml-1 [14C]glucose (Perkin Elmer). Results were normalized for mg of proteins.
Analysis of mitochondrial respiration
Mitochondrial respiration was analyzed using a Seahorse XFe24 workflow. Briefly, 100.000 cells per well were seeded. Twentyfour hours later, cells were treated or not with galanin (50 nM) for 30 min in complete medium. Medium was replaced by Seahorse medium without serum but with 25 mM glucose added [7]. Oligomycin (1 μg/mL), FCCP (3 μM) and a mixture of rotenone (1 μM) plus antimycin A (1 μM) were then added sequentially to, respectively, inhibit the ATP synthase, uncouple oxidative phosphorylation and gauge non-mitochondrial respiration.
Statistical analysis in in vivo and in vitro studies
Data are expressed as mean ± SEM. Comparison between groups was performed by one-way ANOVA followed by a Bonferroni’s post hoc test using GraphPad Prism version 5.00 (GraphPad Software, Inc).
Functional characterization of genes is fundamental to decrypte the biological process in cell subsets driving disease-related manifestations [8]. To infer biological meaning for galanin in cardiac fibroblasts, we firsly generated the RNA-seq gene expression data from galanin-challenged cells. Comparative gene expression analysis identified significant Differentially Expressed Genes (DEGs) that revealed distinct molecular signatures between control and galanin-stimulated cardiac fibroblasts (Figure 1A). Among the top 10 regulated genes, two genes were associated with NFκB inhibition, NFkBIB and NFKBIA and two genes linked to stress-induced cell responses, HSPA1B and EGR1 (Figure 1A). The GO enrichment analysis revealed that the genes with statistically significant differences in expression were mainly associated with regulation of metabolic processes and kinase activity (Figure 1 B-C). The up-regulated genes were dominant components that control metabolic responses mitochondrial translational termination and transcription from RNA polymerase II promoter (Figure 1B), whereas the downregulated genes consisted of genes linked to kinase activity, contranslational protein targeting and cellular lipid metabolic processes (Figure 1C) [9].
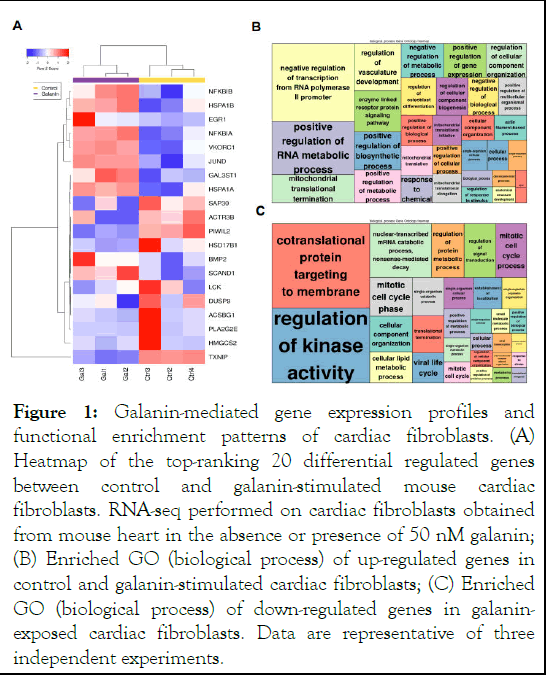
Figure 1: Galanin-mediated gene expression profiles and functional enrichment patterns of cardiac fibroblasts. (A) Heatmap of the top-ranking 20 differential regulated genes between control and galanin-stimulated mouse cardiac fibroblasts. RNA-seq performed on cardiac fibroblasts obtained from mouse heart in the absence or presence of 50 nM galanin; (B) Enriched GO (biological process) of up-regulated genes in control and galanin-stimulated cardiac fibroblasts; (C) Enriched GO (biological process) of down-regulated genes in galaninexposed cardiac fibroblasts. Data are representative of three independent experiments.
We further tested the predicted metabolic significance of galanin in in vitro and in vivo models of fibroblast activation triggering fibrotic remodeling. To directly assess whether galanin affects metabolic status of cultured cardiac fibroblasts, we measured the mitochondrial respiratory bioenergetics in naive and TGFβ-exposed cardiac fibroblasts using Seahorse extracellular flux analyser. As shown in Figure 2A-D, galaninmediated suppression of TGFβ-induced excessive collagen production and α-SMA expression was associated with marked changes in Oxygen Consumption Rate (OCR) under basal and maximal conditions [10]. Strikingly, TGFβ-challenged fibroblasts exhibited an abnormal mitochondrial respiration, loss of ATP level and glycolytic activity, whilst treatment of cells with galanin promoted both mitochondrial basal and maximal respirations, ATP generation and glycolytic capacity (Figure 2 E-H).
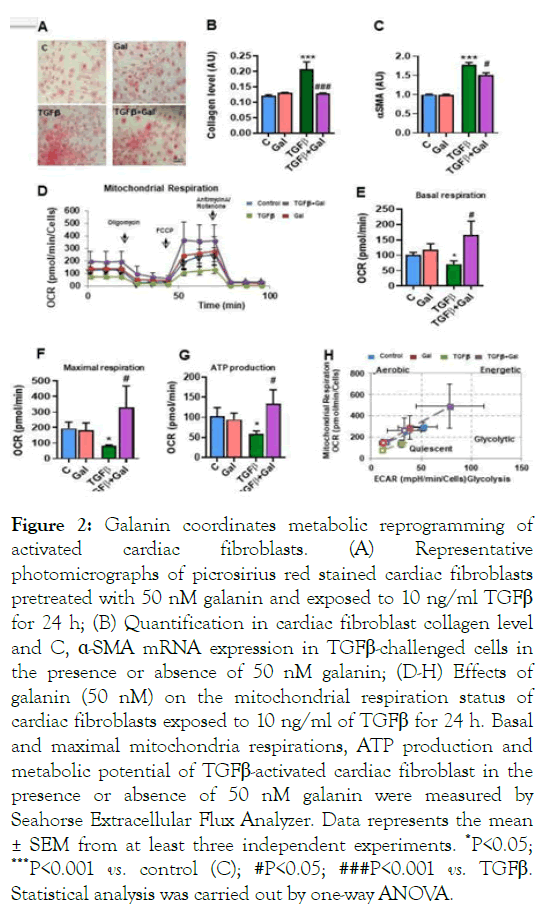
Figure 2: Galanin coordinates metabolic reprogramming of activated cardiac fibroblasts. (A) Representative photomicrographs of picrosirius red stained cardiac fibroblasts pretreated with 50 nM galanin and exposed to 10 ng/ml TGFβ for 24 h; (B) Quantification in cardiac fibroblast collagen level and C, ÃÂÂÂ-SMA mRNA expression in TGFβ-challenged cells in the presence or absence of 50 nM galanin; (D-H) Effects of galanin (50 nM) on the mitochondrial respiration status of cardiac fibroblasts exposed to 10 ng/ml of TGFβ for 24 h. Basal and maximal mitochondria respirations, ATP production and metabolic potential of TGFβ-activated cardiac fibroblast in the presence or absence of 50 nM galanin were measured by Seahorse Extracellular Flux Analyzer. Data represents the mean ± SEM from at least three independent experiments. *P<0.05; ***P<0.001 vs. control (C); #P<0.05; ###P<0.001 vs. TGFβ. Statistical analysis was carried out by one-way ANOVA.
We next evaluated the capacity of galanin to affect the metabolic status of fibrosis-remodelled hearts in a mouse model of HF established by AB-induced pressure overload. As shown in Figure 3A-B, mice subjected to AB for 5 weeks exhibited characteristics of abnormal tissue fibrotic remodeling associated with upregulation of fibrotic markers including collagen type I, type III, α-SMA and TGFβ1 compared to sham-operated control group (Figure 3C-F). The analysis of metabolic profiles of fibrosis-remodelled heart revealed a significant increase in fatty acid oxidation and reduction in myocardial glucose consumption compared to sham control group (Figure 4 A-B). In addition, we found reduced myocardial mitochondrial biogenesis in AB-challenged heart as depicted by decreased expression of NRF1 and PGC1α (Figure 4 C-D) [11]. In contrast, galanin treatment (10 μg/kg/day, i.p.) started 1 week after the onset of pressure overload blunted AB-mediated tissue fibrotic remodeling (Figure 4 A-F) and promoted glucose oxidation (Figure 4A). Importantly, galanin-dependent decrease in fatty acid oxidation was associated with improvement of mitochondrial biogenesis in AB-challenged hearts (Figure 4 BD). Furthermore, we found that galanin-mediated prevention of AB-mediated fibrotic and metabolic remodeling was associated with reduced inflammatory relief as shown by decreased number of CD68-positive cells in cardiac tissue from AB-challenged mice (Figure 4E-F). Galanin-dependent attenuation of myocardial inflammatory events was confirmed by the decreased infiltration of the fibrous cap by inflammatory cells in H and E-stained cardiac tissue from fibrosis-remodelled hearts (Figure 4G) [12].
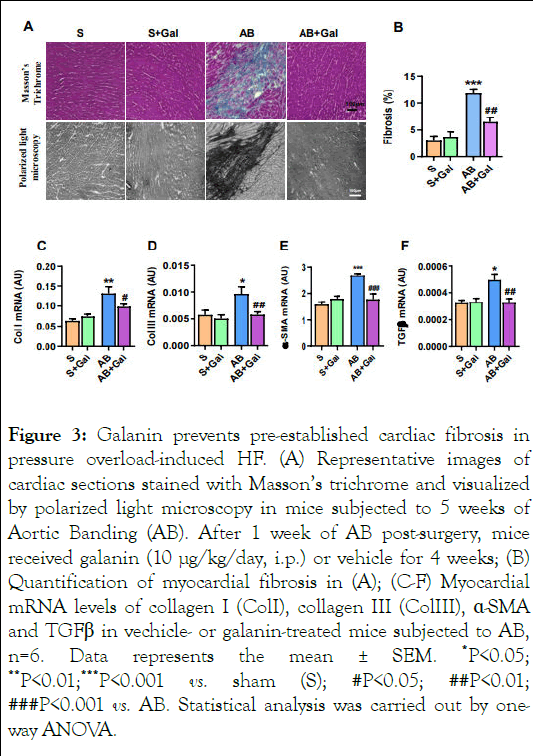
Figure 3: Galanin prevents pre-established cardiac fibrosis in pressure overload-induced HF. (A) Representative images of cardiac sections stained with Masson’s trichrome and visualized by polarized light microscopy in mice subjected to 5 weeks of Aortic Banding (AB). After 1 week of AB post-surgery, mice received galanin (10 μg/kg/day, i.p.) or vehicle for 4 weeks; (B) Quantification of myocardial fibrosis in (A); (C-F) Myocardial mRNA levels of collagen I (ColI), collagen III (ColIII), ÃÂÂÂ-SMA and TGFβ in vechicle- or galanin-treated mice subjected to AB, n=6. Data represents the mean ± SEM. *P<0.05; **P<0.01;***P<0.001 vs. sham (S); #P<0.05; ##P<0.01; ###P<0.001 vs. AB. Statistical analysis was carried out by oneway ANOVA.
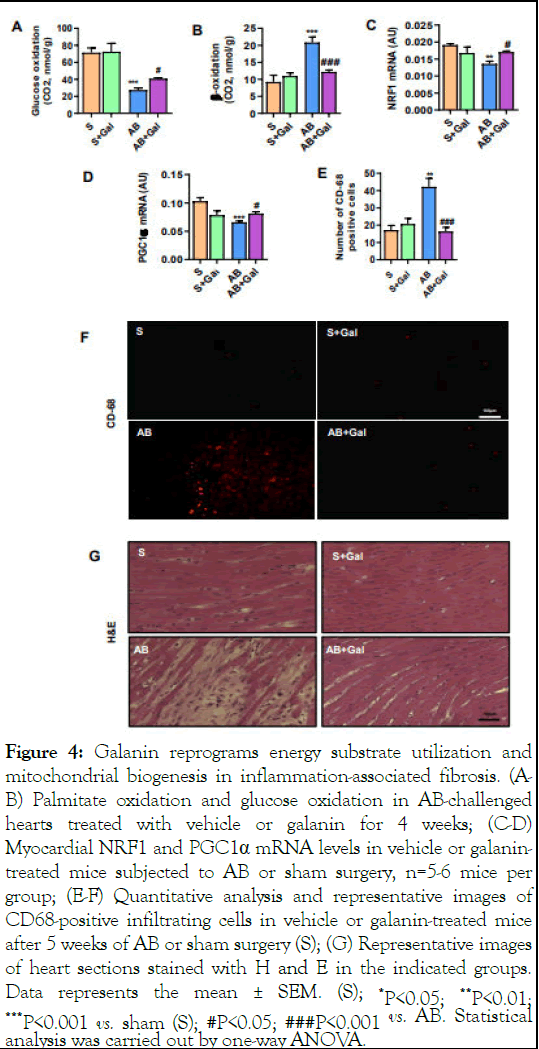
Figure 4: Galanin reprograms energy substrate utilization and mitochondrial biogenesis in inflammation-associated fibrosis. (AB) Palmitate oxidation and glucose oxidation in AB-challenged hearts treated with vehicle or galanin for 4 weeks; (C-D) Myocardial NRF1 and PGC1α mRNA levels in vehicle or galanintreated mice subjected to AB or sham surgery, n=5-6 mice per group; (E-F) Quantitative analysis and representative images of CD68-positive infiltrating cells in vehicle or galanin-treated mice after 5 weeks of AB or sham surgery (S); (G) Representative images of heart sections stained with H and E in the indicated groups. Data represents the mean ± SEM. (S); *P<0.05; **P<0.01; ***P<0.001 vs. sham (S); #P<0.05; ###P<0.001 vs. AB. Statistical analysis was carried out by one-way ANOVA.
In HF phenotype, defects in the structural organization of the myocardium closely link to cardiac performance. We next examined the parameters of left ventricular function by echocardiography in mice using high-frequency ultrasound. Analysis of cardiac function revealed that fibrotic phenotype of AB-challenged mice was associated with impaired systolic dysfunction and structural abnormalities as evidenced by decrease in EF and FS and increase in IVSs and LVIDs, respectively (Figure 5A-E) [13]. As shown in Figure 5A-E, galanin treatment significantly decreased both IVSs and LVIDs and improved systolic function compared to AB-challenged group.
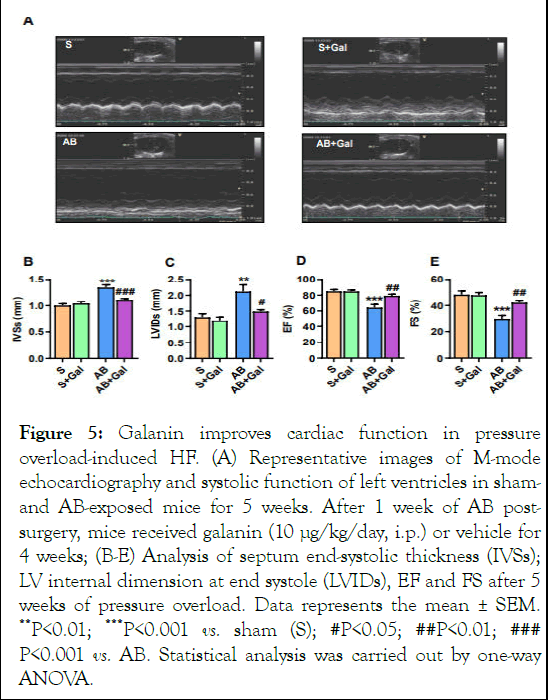
Figure 5: Galanin improves cardiac function in pressure overload-induced HF. (A) Representative images of M-mode echocardiography and systolic function of left ventricles in shamand AB-exposed mice for 5 weeks. After 1 week of AB postsurgery, mice received galanin (10 μg/kg/day, i.p.) or vehicle for 4 weeks; (B-E) Analysis of septum end-systolic thickness (IVSs); LV internal dimension at end systole (LVIDs), EF and FS after 5 weeks of pressure overload. Data represents the mean ± SEM. **P<0.01; ***P<0.001 vs. sham (S); #P<0.05; ##P<0.01; ### P<0.001 vs. AB. Statistical analysis was carried out by one-way ANOVA.
Galanin orchestrates cardiac fibroblast metabolic responses through FoxO1 pathways
We further decrypted the molecular mechanism underlying the galanin-induced metabolic decisions in activated cardiac fibroblasts. Analysis of potential signaling pathways revealed reduced expression level of FoxO1, a key factor in metabolic and oxidative stress-related cellular responses, in TGFβ-exposed cultured mouse cardiac fibroblasts (Figure 6A-B) and in ABchallenged hearts (Figure 6C) compared to control conditions. Interestingly, galanin treatment markedly increased FoxO1 expression in both activated fibroblasts (Figure 6A-B) and ABremodelled heart (Figure 6C). These observations prompted us to examine whether FoxO1 expression level is involved in galanin-mediated antifibrotic and metabolic responses in TGFβ- challenged cells [14]. We next compared galanin-induced effects on collagen production and mitochondrial respiration in scramble (control) and FoxO1 siRNA transfected fibroblasts exposed to 10 ng/ml TGFβ. In activated cardiac fibroblasts, knockdown of FoxO1 resulted in the abolishment of the inhibitory effects of galanin on TGFβ-induced collagen accumulation (Figure 6 D-E). In addition, we evaluated the bioenergetic potential of TGFβ-activated cardiac fibroblasts using siRNA targeting FoxO1. As shown in Figure 7A-C, TGFβ treatment alone resulted in decreased mitochondrial basal and maximal respiration linked to ATP loss in control siRNAtransfected cardiac fibroblasts. In contrast, galanin treatment of TGFβ-activated cardiac fibroblasts promoted basal respiration, maximal respiration capacity and ATP production (Figure 7AC). Strikingly, FoxO1 knockdown resulted in the abolishment of galanin-mediated stimulation of mitochondrial respiration potential and energy generation in activated cardiac fibroblasts (Figure 7A-C) suggesting that galanin-mediated metabolic reprogramming of pro-fibrotic phenotype in cells is FoxO1 dependent [15].
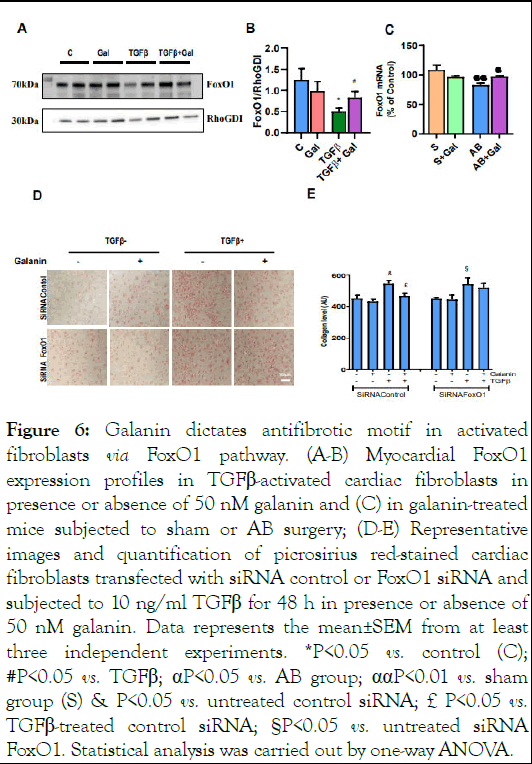
Figure 6: Galanin dictates antifibrotic motif in activated fibroblasts via FoxO1 pathway. (A-B) Myocardial FoxO1 expression profiles in TGFβ-activated cardiac fibroblasts in presence or absence of 50 nM galanin and (C) in galanin-treated mice subjected to sham or AB surgery; (D-E) Representative images and quantification of picrosirius red-stained cardiac fibroblasts transfected with siRNA control or FoxO1 siRNA and subjected to 10 ng/ml TGFβ for 48 h in presence or absence of 50 nM galanin. Data represents the mean±SEM from at least three independent experiments. *P<0.05 vs. control (C); #P<0.05 vs. TGFβ; αP<0.05 vs. AB group; ααP<0.01 vs. sham group (S) & P<0.05 vs. untreated control siRNA; £P<0.05 vs. TGFβ-treated control siRNA; §P<0.05 vs. untreated siRNA FoxO1. Statistical analysis was carried out by one-way ANOVA.
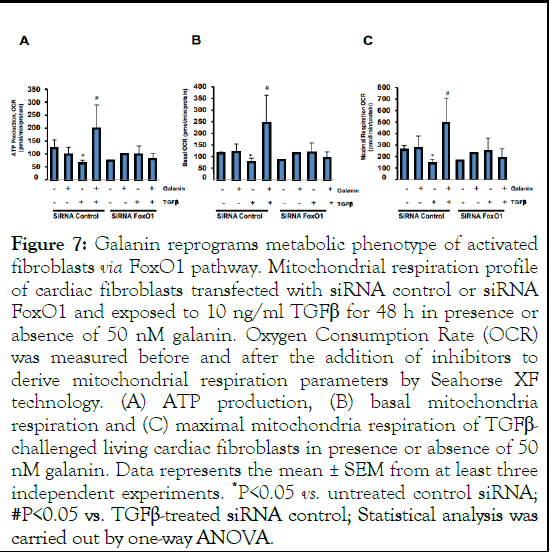
Figure 7: Galanin reprograms metabolic phenotype of activated fibroblasts via FoxO1 pathway. Mitochondrial respiration profile of cardiac fibroblasts transfected with siRNA control or siRNA FoxO1 and exposed to 10 ng/ml TGFβ for 48 h in presence or absence of 50 nM galanin. Oxygen Consumption Rate (OCR) was measured before and after the addition of inhibitors to derive mitochondrial respiration parameters by Seahorse XF technology. (A) ATP production, (B) basal mitochondria respiration and (C) maximal mitochondria respiration of TGFβ- challenged living cardiac fibroblasts in presence or absence of 50 nM galanin. Data represents the mean ± SEM from at least three independent experiments. *P<0.05 vs. untreated control siRNA; #P<0.05 vs. TGFβ-treated siRNA control; Statistical analysis was carried out by one-way ANOVA.
Characterization of the precise nature of bioactive peptides and the decryption of the cell-specific activities are essential and represent the critical prerequisite for the development and discovery of novel therapeutic agents for human diseases. The present study uncovered metabolic motifs of fibroblast transition from naive to reactive profibrotic phenotype promoting aberrant fibrosis and identified galanin as a checkpoint regulator of fibroblast metabolism in HF phenotype. Using mouse model of chronic pressure overload-induced HF, we found that galanin redirects cardiac energy metabolism from fatty acid oxidation towards glucose oxidation in fibrosisremodelled heart. We provide the first evidence that galanin calibrates fibroblast mitochondrial respiratory activity and blunts collagen accumulation through the FoxO1 pathway [16]. Given the central place of fibroblasts in fibrosis-predisposed cardiac dysfunction, these data indicate a novel mechanistic link between metabolic reprogramming of activated cardiac fibroblasts and fibrosis progression in HF phenotype. These findings have important implications for the modulation of cardiac fibroblast behaviors in a pathological scenario and provide a new metabolism-oriented angle for therapeutic intervention to tackle cardiac fibrosis.
Comparative transcriptomics can decode the function of genes in a complex pathological scenario. In the present study we executed a comprehensive RNA-seq benchmarking experiment to uncover the functional significance of peptide galanin in cardiac fibroblasts. Transcriptome and gene ontology enrichment analysis revealead genes mainly involved in metabolic processes that affect fibroblast behaviour in the presence of galanin. The main function of cardiac fibroblasts is to support the structural integrity of myocardial tissues by continuously secreting ECM components, however metabolic rewiring of cardiac fibroblasts remains obscure [17]. Abnormal ECM accumulation is both a primary cause and consequence of fibroblast activation priming tissue fibrotic remodeling. Specifically, TGFβ1 is a powerful fibroblast behavior modulator, favoring the incorporation of α- SMA within cytoskeletal stress fibers, a typical phenotype in aberrant fibrotic reorganization of cardiac tissue. As a result of fibroblast activation-induced ECM reprogramming, the inflammatory motifs contribute to the initiation and progression of tissue fibrosis. In a mouse model of pressure overload-induced HF, we demonstrated that myocardium manifests a robust fibrotic phenotype linked to α-SMA-dominated and inflammatory relief. However, galanin treatment prevented preestablished cardiac fibrosis and appearance α-SMA-positive myofibroblastic cell population [18]. Importantly, we initiated galanin treatment after 1 week of AB-induced cardiac damage suggesting that post-treatment with galanin may be promising strategy to combat reactive fibrotic reprogramming in clinical practice. In addition, we foundt that in fibrosis-challenged hearts, galanin reduced myocardial infiltration of CD68-positive macrophages in mice indicating galanin-dependent antiinflammatory signature in fibrosis-remodelled heart. Macrophage infiltration is known to aggravate cardiac remodeling and is a significant contributor to inflammatory signature of cardiac dysfunction. Several studies reported the strong association between the level of proinflammatory factors and cardiac performance in HF progression. Further efforts will be required to explore clinical translation of anti-fibro-inflammarory potential of galaninin preclinical and clinical models.
Abnormal energy metabolism is one of the most important early events in failing hearts. Interestingly, we pinpointed that galanin promotes glucose oxidation in fibrosis-remodelled hearts suggesting that metabolic signature of aberrant fibrotic tissue reorganization reflects the predominance of carbohydrates as substrates for energy provision in oxygen-deficient environment. When the normal heart is exposed to an oxygen rich environment, energy substrate metabolism is mainly assumed by oxidation of fatty acid as the heart displays privileged mitochondrial oxidative capacity, with fatty acid contributing to 80 ~70% of the total ATP generation. Mitochondria are massively occupe cardiac tissue and point as the primary deliver of ATP. In cardiac cells, the primary function of the mitochondria is to manage the high energy requirement of the heart in both physiological and pathological scenarios. In activated cardiac fibroblasts we uncovered that galanin regulates mitochondrial stress and energy metabolism in TGFβ-activated cardiac fibroblasts unlocking an unknown role of galanin in metabolic orchestration of fibroblast conversion to reactive myofibroblastic phenotype dictating fibrosis resolution [19]. Since the human heart has limited regenerative capacity, galanin-mediated optimization of energy metabolism in cardiac fibroblasts may counteract the untoward effects of abnormal cardiac remodeling linked to loss of cardiac performance.
Although posing role of activated cardiac fibroblast in tissue fibrotic remodeling, molecular mechanisms that may link the fibroblast metabolic status to fibrosis progression remain unknown. We uncovered that galanin prevented aberrant collagen accumulation and metabolic defects in HF phenotype through the FoxO1 pathway. FoxO1, a prominent member of the forkhead box family and subfamily O of transcription factors, orchestrates vital metabolic functions including metabolism, oxidative stress response, immune homeostasis and hypertrophy [20]. We found that in TGFβ-challenged conditions, knockdown of FoxO1 resulted in the suppression of the inhibitory effects of galanin on TGFβ-mediated collagen accumulation and mitochondrial biogenesis suggesting that galanin coordinates metabolic and profibrotic cell decisions via FoxO1 pathway.
Together, these findings unravel a previously unknown role of galanin in metabolic rewiring of aberrant cardiac fibroblast activation dictating fibrosis resolution. We found that fibrosisremodelled hearts exhibit distinct metabolic phenotypes that naive and aberrantly activated cardiac fibroblasts dictating fibrosis resolution. We further identify galanin as a metabolic checkpoint in fibroblast-to-myofibroblast transition in HF phenotype and provide a new metabolism-based angle for therapeutic intervention in cardiac fibrosis.
Lesia Savchenko: Investigation, methodology, writing-review and editing; Rodica Anesia: Investigation, methodology; Andrei Timotin: Formal analysis, investigation, methodology; Dimitri Marsal: Investigation; Alexander Kel: RNAseq analysis; Ilenia Martinelli: Formal analysis, investigation, review and editing; Roncalli Jerome: Project administration, validation; Oksana Kunduzova: Writing-original draft, conceptualization; Validation, funding acquisition, writing-original draft, writingreview and editing.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
We thank Mr. Denis Calise for performing experimental heart surgery.
This study was supported by the French National Institute of Health and Medical Research (INSERM) and Region Midi- Pyrenees.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Savchenko L, Anesia R, Timotin A, Kel A, Marsal D, Martinelli I, et al. (2025) Galanin Dictates Metabolic Reprogramming in Aberrantly Activated Fibroblasts to Reverse Established Cardiac Fibrosis. Trans Med. 15:344.
Received: 07-Apr-2024, Manuscript No. TMCR-24-30769; Editor assigned: 11-Apr-2024, Pre QC No. TMCR-24-30769 (PQ); Reviewed: 25-Apr-2024, QC No. TMCR-24-30769; Revised: 03-Apr-2025, Manuscript No. TMCR-24-30769 (R); Published: 10-Apr-2025 , DOI: 10.35248/2161-1025.25.15.344
Copyright: © 2025 Savchenko L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.