Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Research Article - (2021)Volume 9, Issue 2
Diabetes mellitus is a metabolic disorder, which leading to male reproductive disorders. Probiotic bacteria are known to be one of the most effective factors in many diseases. This study aimed to evaluate the effect of probiotics Bifidobacterium lactis and lactobacillus casei on sperm parameters and expression of apoptosis BAX and Bcl2 genes in streptozotocin-induced diabetic rats. In this experimental study, 35 adult male Wistar rats were divided into five control groups, diabetic, diabetic treated with: B.lactis, L.casei and both probiotic B.lactis and L.casei. Diabetic was induced by intraperitoneal injection of streptozotocin at a dose of 60 mg/Kg and Probiotic treatment at a dose of 109 cfu/ml for 56 days was done by gavage. One day after the last gavage, blood glucose, serum insulin, sperm parameters and histology and Histomorphometric of testicular tissue were examined. Also, the total amount of RNA was extracted from the treated testicular tissue and analyzed by Real-time PCR. The data’s were evaluated using one-way ANOVA and Tukey, p-value test less than 0.05. In this study, the reduction of sperm parameters, insulin serum levels, Spermatogenesis coefficient and increased blood glucose, Spermatogenesis coefficient testicular tissue degradation was observed significantly in the diabetic group compared to the control group (P<0.001). However, in the treated groups with probiotics, a decrease in blood glucose, an increase in sperm parameters, insulin levels and a decrease in testicular tissue damage was observed in comparison with the diabetic group (P<0.05). Also, the expression of BAX and Bcl2 genes in diabetic groups showed a significant change compared to the control group (P<0.05), but there were no significant changes in probiotic treated groups compared with diabetes. This study demonstrates the effect of improving probiotics receiving diabetic groups on the damage parameters sperm and testicular tissue in diabetic rats. Probiotics may eventually secrete and produce antioxidant and anti-inflammatory compounds and reduce the damaging effects of diabetes.
Diabetes; Bifidobacterium lactis; lactobacillus casei; Sperm parameters; BAX; Bcl2
Diabetes is a disorder in the metabolism of carbohydrates, fats and proteins, caused by the lack of insulin secretion or the decreased sensitivity of the tissues to insulin [1].
Streptozotocin is one of the drugs used to induce both types of diabetes in animal models.
It has many acute and chronic complications on various organs, and disorders such as neuropathy and venous thrombosis in reproductive behaviors produce reproductive tissues.
These complications in male reproductive tissues are reduced the number of sperm, the low quality of seminal fluid, the decrease in testosterone and the reduction of spermatogenesis cells [2].
Also, these testicles are also sensitive to environmental factors causing cell death. Germ cell apoptosis may also occur during no physiological stresses such as ischemia, temperature, radiation, and diabetes [1].
Diabetic patients are prone to problems with their sexual function due to neurological and vascular disorders, including sexual diminished, impotence and infertility [3].
Diabetes mellitus has a variety of functional and structural effects on the reproductive system.
It has been shown that oxidative stress in diabetic patient’s increases due to excessive Reactive Oxygen Activation (ROS), which results in decreased antioxidant defense [4].
Oxidation of lipids, proteins, and other macro-molecules during the development of diabetes falls [5].
Mammalian sperm cells have lipid content with large amounts of unsaturated fatty acids, plasmoglucans, and sphonomolylenes. The lipids in spermatozoa are the main substance for peroxidation [6].
This feature converts testicular tissue into a suitable focus for the production of free radicals due to the peroxidation of lipids, thereby increasing the production of free radicals. Also, cells in this tissue are often divided, resulting in high metabolism, which causes more free radicals, due to the weakness of the antioxidant defense system in the course of diabetes, the accumulation of these radicals in the cell It's too common [5,7].
Free radicals from different intracellular pathways affect the cellular activity and exacerbate apoptosis and damage testicular tissue. The results of the tissue studies showed that due to diabetes, the cells of the spermatozoa and sertoli cells were altered and reduced, which is especially significant in the case of sertoli cells, in addition to decreasing the diameter of the toluene and increasing the diameter of the membrane base was also observed [3].
The low quality of seminal fluid in diabetic patients has been reported, which includes decreased sperm movement, decreased sperm count and increased sperm with abnormal morphology [8].
The induction of DNA destruction in the sperm nucleus and the defect in spermatogenesis affects fertility potential [9].
Oxidative stress contributes to the pathophysiology of impaired spermatogenesis and loss of germ cells [10].
Apoptosis or program cell death plays an important role in the pathogenesis of multiple diseases. Diabetes mellitus causes malformative effects on the male reproductive system and sexual function in animal and human samples of diabetes and increases apoptosis [11].
Apoptotic cell death is significantly increased in the Seminiferous Tubules of Streptozotocin (STZ) induced diabetic mice and rats and is considered one of the major reasons underlying infertility in diabetic animals [12]. Both Reactive Oxygen Species (ROS) overproduction and diminished antioxidant defenses may result from excessive oxidative stress in diabetic testes. If the balance between ROS generation and ROS scavenging systems is lost, superoxide accumulation will eventually cause cellular damage or dysfunction. Additionally, diabetes-mediated oxidative stress can induce apoptosis [13]. Given the adverse effects of oxidative stress, there is growing interest in the use of antioxidants as potentially beneficial therapeutic agents [14].
Due to the side effects of many of the chemical drugs today, many more diseases are associated with the identification of alternate compounds with less side effects or supplements along with drugs with more effects. One of these compounds is beneficial bacteria called probiotics.
The mechanism of action of probiotics is through several mechanisms, including the production of inhibitor compounds, the production of antioxidant compounds, the elimination of toxic compounds, the strengthening of the immune system.
Among the probiotics that are most widely used in studies, Lactobacillus lactis and Bacillus casei are mentioned. Probiotics can reduce the side effects of diabetes on the male reproductive system [15,16]. The purpose of this study is to determine whether probiotics can be used for treatment Streptozotocininduced diabetic complications of the reproductive system in rats.
Animals
In this study, 35 adult male Wistar rats weighing from 250-300 (gr) were purchased from the Pasteur Research Institute and adapted to the environmental conditions for one week prior to the start of the experiment in an animal under laboratory conditions at 20 ± 2 and kept in 12 hours of darkness and 12 hours of brightness and during the duration of the experiment. They were treated by the law of care and use of experimental animals.
Chemical agents
Streptozotocin was purchased as a powder from Sigma Sigma- Aldrich, Germany (St. Louis, MO, USA). NPH insulin was purchased from the Pharmaceutical Mfg. Co., Iran, and probiotics were purchased from takgene company, Iran. Method of preparation and preparation of probiotics. In this study, two probiotic lactobacillus casei (ATCC 39392) and Bifidobacterium lactis (Bb-12) were prepared from single genes and powdered with log10. To prepare the solution for each rat, dissolve 1 g of probiotic in 9 cc distilled water to make probiotic to the log of 109 [17].
Diabetes induction
For diabetic type 1 rats, a dose of 60 mg/kg of streptozotocin (dissolved in 0.1 mm sodium citrate buffer, pH 4.5) was injected into a single dose and a peritoneal animal. For the study of diabetic rats, on the third, fifth and seventh day, the weight and blood glucose were measured. For blood glucose, the rat should be fed fast before the test for 8 hours. A blood sample of the tail vein was obtained and blood glucose was measured through a digital glucometer (Norditalia Elettromedicali S.R.I., Italy). If blood glucose was more than 250 mg/dl, that animal was considered diabetic [17].
Experimental design
The rats were randomly divided into 5 groups (n=7). Control group: rats that were given no drugs and only fed water and food. Diabetic group: Rats that received streptozotocin alone in a dose of 60 mg/kg and intraperitoneally and diabetic groups treated with probiotic by gavage method for 35 days, includes: diabetic (streptozotocin 60 mg/kg)+Lactobacillus asei (109 CFU/ml), diabetic streptozotocin 60 mg/kg +Bifidobacterium lactis (109 CFU/ml), and diabetic streptozotocin 60 mg/kg+mixtures of both probiotic lactobacillus casei, Bifidobacterium lactis (109 cfu/ml) [17]. We showed the experimental plan in Figure 1.

Figure 1: Experimental plan. C: control, D: diabetic, D+L.casei: diabetic+Lactobacillus casei, D+B. Lactis: diabetic+Bifidobacterium lactis, D+L.casei+B. Lactis: Diabetic+Lactobacillus casei, D+B. Lactis: Bifidobacterium lactis.
Sample collection
Glucose levels and body weight were recorded at the regular interval, at the end of the period; Animal weight and testis weight were measured with digital scales. Testis weight index was obtained by dividing the testis weight by multiplying the rat weight by 100 [18].
Animals were anesthetized, and blood samples were taken from their hearts to measure insulin immediately, the testes and epididymis was removed [19]. The epididymal tail was used to evaluate the sperm parameters, left testis for histological studies and the right testis to evaluate the gene expression.
Biochemical analyses of serum insulin
Blood samples were isolated from the heart area to measure the amount of insulin from different test groups and centrifuged at 3000 rpm for 15 minutes. Insulin measurement using Enzyme- Linked Immunosorbent Assay (ELISA) did [20]. The serum insulin levels were determined with insulin ELISA Kit (Shanghai Crystal Day Biotech Co., Ltd., China) according to the manufacturer's protocols.
Sperm preparation and analysis
Cauda Epididymis left was separated and cut in DMEM/F12 containing 10% FBS and was put in incubator at 37°C and 5% CO2 for thirty minutes. The prepared suspension was used for the analysis of sperm parameters (Sperm viability, sperm morphology, sperm count) were evaluated according to the WHO guidelines [19].
Sperm viability
The ratio of a suspension volume of sperm and two volumes of eosin was mixed and a thin expansion of the sample was made on the lam. After spreading dry, 100 sperms were counted using an optical microscope and magnification 400, and the ratio of sperm survival to total sperm was calculated in different groups. In this staining, the head of the live sperm was white; while dead sperms become red.
Sperm counts
To calculate the number of sperm, 10 μl sperm suspension was placed on a new bar lam under a light microscope and after counting, the number of sperms was calculated in milliliters.
Morphological sperm
For sperm morphology, smears were prepared from the samples to examine sperm morphology and stained with the Papanicolaou method. Then, for each sample, 100 sperms were examined by magnifying 400 light microscopes.
Histological examination of the testis
After anesthetizing the rat, Left testis to be fixed for study histology and examination of spermatogenesis in formalin solution 10% and after preparing tissue sections with a thickness of 5 microns Stained with Hematoxylin-Eosin (25).
Historphometric study of testicular tissue
Spermatogenesis coefficient (TDI) and TDI were used to evaluate spermatogenesis in seminal tubes. To evaluate the RI of spermatogonia active percentages of seminal tubules containing four or more TDIs, A row of cells differentiated from seminal spermatogonia was calculated for this purpose. Transverse testis tissue was examined. The ratio of active spermatogonial cells to RI was also calculated to determine the passive SPI percentage in seminal tubes and to evaluate seminal tubules containing sperm to non-sperm tubes. Therefore, three tubes were counted in 4 transverse sections [18].
RNA extraction, cdna synthesis, and real-time PCR
At the end of the treatment, period to examine the expression of the genes BAX and bcl2 by the method of real-time PCR right testis kept frozen immediately in liquid nitrogen and subsequently stored (-80°C) for RNA extraction [21].
RNA extraction
To evaluate the expression of genes BAX and bcl2 at first RAN extraction was performed by Ronx Synchelon solution. The quality and quantity of extracted RNA were determined using electrophoresis and Nanodrop.
cDNA synthesis
Five hundred nanograms of extracted mRNA were reverse transcribed into cDNA using the cDNA synthesis kit (PrimeScriptTM 1st strand cDNA Synthesis Kit, Takara) in 20 μl reaction mixture according to the manufacturer’s instructions. The resulting cDNA kept at -20°C until use.
Real-time PCR
The expression profile of Bcl-2 and BAX genes normalized using β-actin as a housekeeping gene were evaluated. The primer sequences of β-actin, Bcl2 and BAX, genes are shown in Table 1. Primers for Real-time PCR were designed with the Oligo7 software. Real-time PCR was carried out using Applied Biosystems™ Real-Time PCR instruments. 10 μl of SYBR Green PCR master mix, 2 μl of cDNA, and 200 nM primer set were used for amplification in 20 μl reaction mixture. All samples were amplified in triplicates in a 48-well plate and the cycling conditions were as follows: 10 seconds at 95°C, and 40 cycles at 95°C for 5 seconds and 60°C for 30 seconds. Relative Quantification (RQ)=2-ΔΔCt formula was used for assessment of relative expression of genes [22].
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| Ã?actin | 5'-CGT GCG TGA CAT TAA AGA GAA-3' | 5'-CGC TCA TTG CCG ATA GTG AT-3' |
| BAX | 5'-TGC AGA GGA TGA TTG CTG ATG-3' | 5'-GAT CAG CTC GGG CAC TTT AG-3' |
| Bcl-2 | 5'-CCT GGC ATC TTC TCC TTC CAG-3' | 5'-GAC GGT AGC GAC GAG AGA AG-3' |
Table 1: Primers Used in Real-Time Polymerase Chain Reaction Gene Expression Analysis.
Statistical analysis
The results were expressed as mean ± SEM (standard error of the mean). Statistical analysis was performed by SPSS software version 22 (IBM company, SPSS Inc., 2010). One-way Analysis of Variance (ANOVA) followed by post hoc Tukey’s test was used to assess the statistical significance of data between different groups. It was considered significant if P<0.05.
Body weight and testis weight gain
The results of this study showed that diabetic rats had Body weight and volume, weight dimensions and testicular index loss in comparison to the control group (P<0.001).
Decrease in the body weight gain of the diabetic rats compared to controls. Probiotics for 5 weeks caused a significant (P<0.001) increase in the body weight gain in diabetic groups treated with lactobacillus casei, Bifidobacterium lactis. However, these changes were not significant in the diabetic group receiving two doses of probiotics (Table 2).
| Group | Body weight (g) | Testis weight (g) | Testis volume (cm3) | Testis index |
|---|---|---|---|---|
| control | 276.5 ± 11 | 1.71 ± 0.0015 | 1.16 ± 0.098 | 0.61% |
| Diabetes | 197.45 ± 8*** | 0.84 ± 0.0023*** | 0.64 ± 0.31 | 0.42% |
| Diabetes+L. casei | 245.5 ± 11*** | 1.35 ± 0.025*** | 0.97 ± 0.46 | 0.54% |
| Diabetes+B. lactis | 257.3 ± 9***a | 1.76 ± 0.034***a | 0.81 ± 0.01 | 0.68% |
| Diabetes+L. casei+B. L.actis | 236.5 ± 7*** | 1.15 ± 0.028*** | 0.76 ± 0.06 | 0.48% |
| Note: ***; P<0.001, Statistical differences between diabetic and different groups: a; P<0.05. | ||||
Table 2: Body weight, testis weight, Testis volume (cm3) and Testis index of different groups.
Blood glucose and serum insulin levels
Figure 2 shows blood glucose concentrations in rats at different times, before and after treatments. Blood glucose concentration was not significantly different between the groups on day 0.
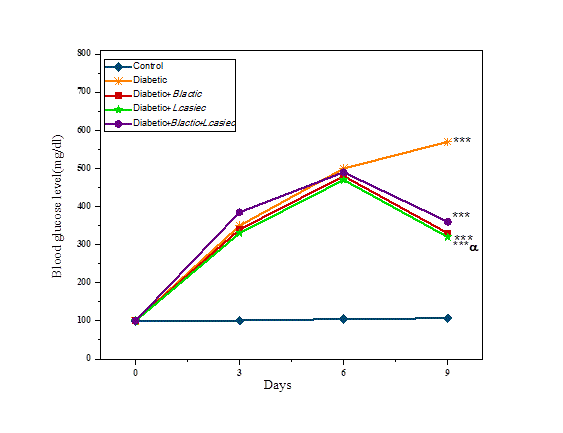
Figure 2: Comparison of changes in glucose levels of rats in different groups.
Statistical differences between control and different groups: ***; P<0.001, Statistical differences between diabetic and different groups: α; P<0.05.
Streptozotocin injection increased blood glucose in diabetic groups after 3, 5, 7 and 56 days compared to control groups (P<0.001).
Consumption of 5 weeks of probiotic in diabetic rats showed a significant decrease in blood glucose levels in diabetic group treated with probiotics L. casei, B. lactic compared to diabetic rats. There was no significant decrease in both probiotic groups in the recipient group.
It was also observed that insulin levels in the diabetic group were significantly reduced compared to the control group (P<0.001), while the treatment groups showed a significant increase compared to the diabetes group (P<0.05) (Figure 3).
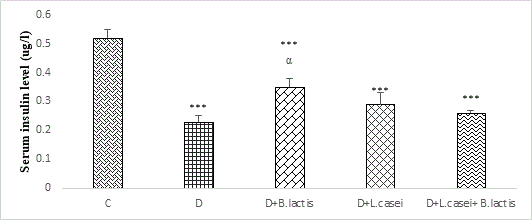
Figure 3: Comparison of changes in insulin levels of rats in different groups.
Statistical differences between control and different groups: ***; P<0.001, Statistical differences between diabetic and different groups: α; P<0.05.
Sperm analysis parameters
Sperm viability: In different groups, it showed that the percentage of viability sperm in the diabetic group was significantly reduced compared with the control group (P<0.001), while the decrease in diabetic groups treated with various probiotics lactobacillus casei and Bifidobacterium lactis was shown in comparison with the control group (P<0.001), while the percentage of viability sperm in treatment groups showed a significant increase compared to the diabetic group (P<0.05) (Figures 4 and 5).
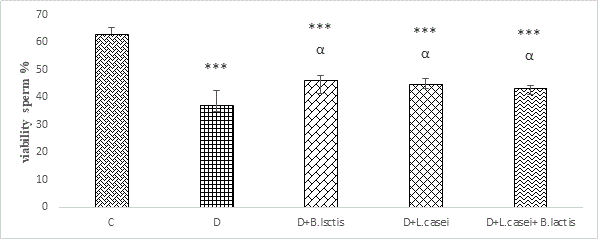
Figure 4: Comparison of changes in the viability sperm of rats in different groups.
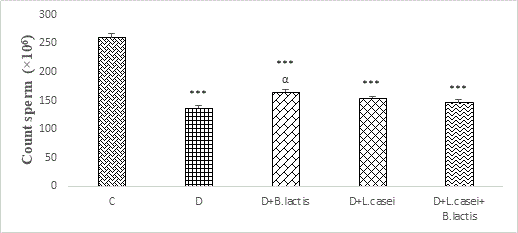
Figure 5: Comparison of changes on count sperm of rats in different groups.
Statistical differences between control and different groups: *; P<0.05, ***; P<0.001, Statistical differences between diabetic and different groups: α; p<0.05.
Sperm count: For sperm count, dilution of 1 to 2 of the mentioned sperm was prepared. The results of sperm counts in different groups showed that the number of sperm in the diabetic and diabetic group treated with probiotics L. casei, B. lactic and the mix of both types of probiotic showed a significant decrease compared to the control group.
Also, in probiotic-treated diabetic groups, the number of sperm increased compared to the diabetic group, but it was not significant (Figure 5).
Statistical differences between control and different groups: ***; P<0.001, Statistical differences between diabetic and different groups: α; P<0.05.
Sperm morphology: The results of sperm morphology showed a significant increase in sperm percentage with abnormal morphology between the diabetic group and diabetic group treated with probiotics compared to control group (P<0.001).
On the other hand, in diabetic groups receiving probiotics, the number of sperm with abnormal morphology was reduced in comparison with the diabetic group, but it is not significant.
On the other hand, in diabetic groups receiving probiotic B.lactis and probiotic mixtures of L. casei and B. lactis, the number of sperm with non-morphology decreased in comparison to the diabetic group, but it was not significant, while in the diabetic group treated with probiotic B. lactis decreased was observed to be significant P<0.05 (Figures 6 and 7).
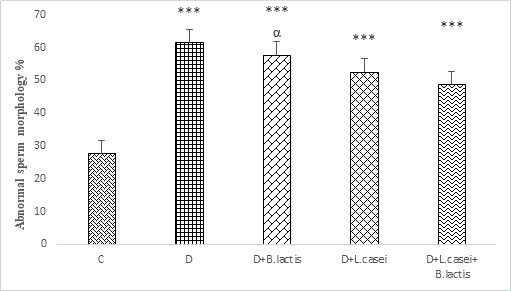
Figure 6: Comparison of changes in sperm morphology of rats in different groups.
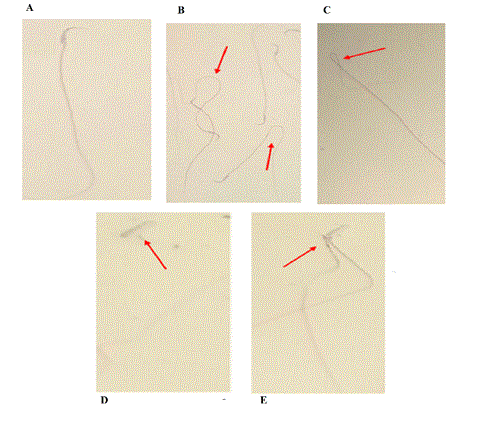
Figure 7: The appearance of abnormal sperm. Various abnormal sperm shapes are observed under a light microscope. A: Normal sperm, B: Curved sperm, C: Sperm with a tufted tail, D: Sperm without tails, E: Sperm with two breasts, Stained with Papanicolaou, 400 Ã? magnification. Microphotographs illustrating morphologically normal sperm and various sperm defects.
Statistical differences between control and different groups: ***; P<0.001, Statistical differences between diabetic and different groups: α; P<0.05.
Histological and histomorphometric observations
In the histological sections of the testis of rats in five groups, the number of Sertoli cells, spermatogonial cells, spermatocytes andspermatids, in the diabetic group showed a significant change compared to the control group, while in diabetic groups treated with lactobacillus casei, Bifidobacterium lactis and probiotic mix, these changes were lower compared to the diabetic group (Table 1).
On the other hand, an increase in the thickness of the basemen membrane was observed in the diabetic group as compared to the control group. It was also shown that the atrophy of seminiferous tubules in diabetic rats increased significantly, which was less observed in diabetic rats treated with probiotics (Table 3).
| Â Group | Reduced number of Sertoli cell | Reduced number of spermatocytes |
Reduced number of spermatids |
Atrophic tubules | Increased thickness of the basement membrane |
|---|---|---|---|---|---|
| control | --- | --- | --- | --- | --- |
| Diabetes | +++ | +++ | +++ | +++ | +++ |
| Diabetes+L. casei | ++ | ++ | ++ | ++ | ++ |
| Diabetes+B. lactis | + | + | + | + | + |
| Diabetes+L. casei+B. lactis | ++ | ++ | ++ | ++ | ++ |
Table 3: Comparison of changes in testicular tissue in different groups. Each group consisted of seven animals. -Absent, + slight, ++moderate, +++ severe.
The results showed that the probiotics had a significant effect on spermiogenesis in diabetic groups compared to diabetic nodules (p<0.05) (Table 4).
| Group | Positive TDL (%) | Positive SPI (%) | Positive RI (%) |
|---|---|---|---|
| control | 87.56 ± 6.78 | 83.6.54 | 78.32 ± 10.12 |
| Diabetes | 35.45 ± 3.46 | 32.25 ± 10.1 | 31.34 ± 6.72 |
| Diabetes+L. casei | 76.18 ± 4.54 | 71.17 ± 1.13 | 65.3.44 ± 5.63 |
| Diabetes+B. lactis | 63.18 ± 3.4 | 65.30 ± 2.34 | 64.21 ± 2.07 |
| Diabetes+L. casei+B. lactis | 57.56 ± 4.61 | 68.63 ± 1.83 | 61.41 ± 2.32 |
Table 4: Comparison of spermiogenesis coefficients in study groups.
mRNA expression of BAX and bcl-2 genes
RT-PCR was performed to investigate the effect of probiotic B.lactis and L.casei on the mRNA expression of BAX and Bcl-2 genes in testicular tissue of diabetic rats.
In this study, the increased expression of BAX mRNA expression and reduction of bcl2 mRNA expression in diabetic and diabetic groups treated with probiotics B.lactis and L.casei was compared with the control group (Table 5).
| Group | BAX | Bcl2 | BAX/Bcl-2 ratio |
|---|---|---|---|
| Control | 0.42 ± 027 | 1.26 ± 0.42 | 0.33 ± 0.21 |
| Diabetes | 1.76 ± 0.65* | 0.74 ± 0.16* | 2.37 ± 0.57 |
| Diabetes+L. casei | 1.37 ± 0.71* | 0.87 ± 019* | 1.57 ± 0.16 |
| Diabetes+B. lactis | 1.49 ± 0.09* | 0.82 ± 0.45* | 1.81 ± 0.24 |
| Diabetes+L. casei+B. lactis | 1.51 ± 0.65* | 0.77 ± 0.36* | 1.96 ± 0.36 |
| Note: *; P<0.05 | |||
Table 5: mRNA expression of BAX and Bcl-2 genes analyzed by RT-PCR.
However, no changes were observed in the expression of BAX mRNA and bcl2 mRNA in diabetic groups treated with B.lactis, L.casei probiotics and a mixture of both probiotics compared to diabetic rats (Table 5).
In this study effect of probiotics Bifidobacterium lactis and lactobacillus casei in streptozotocin-induced diabetic rats were evaluated by measuring testis weight, sperm parameters (viability, morphology, count) and testicular histological examination.
Also, the induction of apoptosis in the testicles by determining the changes in BAX, Bcl-2 genes expression was investigated using the Real Time-PCR method.
It was observed that in diabetic rat receiving probiotics lactobacillus casei and Bifidobacterium lactis compared to the diabetic group, the weight of the rat, testis weight, blood glucose, serum insulin, sperm parameters, and testicular tissue showed a relative improvement, also the expression of apoptotic genes BAX And bcl2 in the diabetic group show a significant difference compared to the control group, but the groups receiving probiotics did not show a significant difference compared to the diabetic group.
In STZ-induced diabetes models, male reproductive system damage is associated with increased oxidative stress in testicular tissue and thus reduces the effectiveness of antioxidant responses; inducing diabetes in animals reduces body weight and decrease testis volume [23]. Our studies also agree on the agreement with previous studies.
Also to investigate the spermatogenesis process of RI, SPI coefficients and TDI in different groups. The results showed that these coefficients were significantly decreased in the diabetic group compared to the control group, which is a reason for the decrease in sperm production, whereas in the diabetic groups treated with probiotics Bifidobacterium lactis and lactobacillus casei showed a significant increase in spermatogenesis coefficients.
Diabetes-induced oxidative stress can damage testicular lymphoid cells and apoptosis. Sertoli cells are involved in maintaining the integrity of spermatogenesis, germ cell maturation and, at the same time, germ cells to control spermatogenesis in the testicular tissue. Decreased testosterone and immature germ cell shedding and Sertoli cell injury may disrupt spermiogenesis, which is in agreement with the findings of this study suggesting a decrease in Sertoli cells and spermatogenesis coefficients in the diabetic group. Histological studies showed that there was a significant decrease in sertoli cells with a significant disruption of germ cells in the diabetic group. Therefore, the reduction of tubular differentiation coefficient and the replacement coefficient of spermatogonial cells in the diabetic group compared to the control group can be justified [18].
Previous studies have shown that testicular size is highly correlated with the number of Sertoli cells and the production of sperm. In other words, the size of testicles reflects the number of germ cells present in it. The inhibition of spermatogenesis by removing the pituitary gland reduces testicular weight significantly [7].
One of the most important pathways associated with hyperglycemia is the induction of oxidative stress, which has been proven in several studies [24].
In hyperglycemia, glucose metabolism also increases the production of these radicals, causing damage to various macromolecules such as DNA, proteins, carbohydrates, and lipids.
Hyperglycemia, in addition to the production of free radicals, inhibits the antioxidant system of the body, including the reduction of glutathione, which is also due to an imbalance between oxidants and antioxidants and intensifies the induction of any evidence in the clinical and experimental study. There is evidence that there is a close relationship between hyperglycemia, the induction of oxidative stress, and complications from diabetes [25].
According to studies, the cause of the decrease in glucose by streptozotocin injection has shown that the results of the action of streptozotocin toxicity in the beta-pancreatic cell are due to a significant change in cellular DNA. Experiments have shown that the main cause of demyelination and death these cells are the alkylation of their DNA. The alkylation property of these cells is related to the urea nitrate portion, with most of the guanine O6 position in the DNA being exposed to methylation [25].
The low quality of seminal fluid in diabetic patients has also been reported, which includes decreased sperm motility [26].
Reduced sperm count and increased sperm count with abnormal shape (Leng et al., 2004). In this study, it was also shown.
The reduction of sperm density should be due to the severe effect of glucose increase in the last stages of spermatogenesis.
According to the theory of free radicals, the imbalance between pro-oxidants and antioxidants ultimately causes oxidative damage in cellular processes and reduces steroidogenesis in cells Leydig [27].
In the study of Lotfi et al., induced diabetes with STZ, serum testosterone levels, testicular weight, the diameter of the tubes, and total sperm count decreased [28].
A study by La Vignera has shown that oxidative stress and free radicals play a major role in reducing reproductive parameters such as sperm and fertility in type-2 diabetes [26].
Diabetes seems to produce testicular changes through the formation of apoptosis, atrophy of the intubation tubes, the decrease in the diameter of the seminal tubes, and the reduction of the cellular complex of spermatogenesis, and has a deleterious effect on the production of spermatozoa and spermatogenesis [12,29]. In this study, the reduction of spermatogenesis in the diabetic group was shown.
Important role of active oxygen species in the development of testicular tissue defects and abnormalities in diabetic rats has been reported [30].
Mammalian sperm cells have lipid content with large amounts of unsaturated fatty acids, plasma lungs, and sphongomolilin. The spermatozoa are the main source of peroxidation [31].
This property converts the testicular tissue into an appropriate focal region for the production of free radicals due to the peroxidation of lipids, thereby increasing the production of free radicals. In addition, the cells in this tissue are alternately divided into the result is high metabolism, which results in the production of more free radicals, due to the weakness of the antioxidant defense system in the course of diabetes, the accumulation of these radicals in the cell is more than usual. The free radicals produced from different intracellular pathways affect cellular activity and exacerbate apoptosis and tissue damage in the testes.
Probiotics are non-pathogenic living microorganisms that, if consumed enough, have beneficial effects on the health of the host [32].
The proposed mechanism is to justify the ability of probiotics to protect their host, to resist colonization and to inhibit pathogenic pathogens. Also, by producing organic acids such as acetate, probiotic nate, butyrate, bacterial compounds make competitive containment in the position connections of bacteria on the epithelial surface of the intestine with pathogenic species and immune system enhancement can protect the host against pathogens [30].
Probiotics also have the ability to produce antioxidant metabolites, the ability to regulate the activity of host antioxidants, increase the level of antioxidant metabolites in the host, increase the antioxidant enzymes in the host by adjusting some signaling pathways Such as PKC, MAPK, NFK-B, Nrf2, reducing the production of ROS by reducing the activity of free radical production enzymes can regulate the level of antioxidants in their host [15].
Since in diabetic group, the possible reasons for the increased free radicals in diabetic patients due to the increased activity of oxygen production as a result of glycosylation is a very important factor in reducing lipid peroxidation in diabetic patients with glucose regulation. Preventing lipid peroxidation reduces the progression of diabetes and its complications [31].
In this study, in diabetic groups receiving probiotics B.lactis and L.casei compared to the diabetic group, relative recovery in various factors such as rat weight, testicular weight, glucose level, sperm parameters, and testicular tissue were shown. Probably because of the mechanisms mentioned. Also, in a study conducted by Yadav et al, on mice, fermented milk with Lactobacillus acidophilus and Lactobacillus caesium could begin to disturb glucose metabolism in mice fed with a high fructose diet. Delayed and anti-diabetic effects [33].
The intestinal microflora composition is effective in determining the amount of inflammation involved in diabetes so that when the balance in the intestinal microflora is eliminated, the ratio of the gram-positive bacteria to the gramnegative in the intestine decreases, resulting in the absorption of lipopolysaccharides and other pro-inflammatory molecules increase their circulation to the bloodstream, which increases the secretion of cytokines, the activity of macrophages, and inflammation in the body. Inflammatory cytokines can impair the function of receptors Insulin and thus insulin resistance, also induces apoptosis of beta-pancreatic cells, decreasing secretion insulin is made by cells [34].
In general, intestinal microflora imbalance plays a role in the progression of diabetes. The concentration of lipopliscicides in plasma is inversely related to the Bifidobacterium population in the intestine. Bifidobacterium can reduce intestinal endotoxin levels. It improves the mucosal level of the intestine and reduces bowel inflammation [34].
Yadav and colleagues investigated the anti-diabetic effects of Dahi's probiotic product containing Lactobacillus caesium and Asfophilus on fructose-diabetic rats and showed that probiotics used to reduce glucose and glucose intolerance.
Harris and colleagues showed that Lactobacillus acidophilus reduces free radicals and reactive oxygen species. These reactive compounds disrupt nitric acid levels in diabetic rats. Important nitric oxide secreting hormones and stimulating the immune system. In other words, Lactobacillus acidophilus has been shown to reduce blood glucose and modify nitric oxide in alloxaninduced diabetic rats [35].
It is likely that the cause of blood loss in probiotics receiving groups is related to the antioxidant property, which prevents the destruction of beta-Langerhans cells and thus reduces serum glucose [36].
Probiotics are therefore likely to reduce the production of free radicals and decrease the level of oxidative stress in diabetic patients by decreasing blood glucose levels through the mechanisms mentioned. Probiotics significantly inhibited lipid peroxidation and formation of nitric oxide in the pancreatic tissue, thereby reducing the oxidative damage induced and increasing the antioxidants such as glutathione, glutathione peroxidase, and superoxide dismutase activity. Reducing the oxidative stress caused by the use of probiotics is due to their effect on the increased level of glutathione recovered and the hydroxyl and superoxide radicals [36].
The proapoptotic BAX and antiapoptotic bcl-2 are two critical molecules involved in cell death, and the ratio of BAX/Bcl-2 is the denominator that decides whether cells will undergo apoptosis. The STZ-induced diabetic rats had increased BAX (therefore, increased ratio of BAX/bcl-2) and decreased bcl-2 expression in their testis tissues.
Another part of this study was to investigate the expression of BAX and Bcl-2 genes by the real-time method, our results showed that the mRNA expression BAX was significantly higher in the diabetic group than that of the control group; but, in the diabetic group treated with Probiotics B.lactis and L. casei, they had lower levels of BAX as compared with the diabetic group but no significant changes.
Also, the mRNA level of bcl-2 were significantly decreased in the diabetic group compared with the control group, in the diabetic group receiving probiotics, they were increased in comparison with the diabetic group but no significant difference.
A number of studies have shown that the inflammation induced by hyperglycemia is the main mechanism of the pathogenesis of cell apoptosis and diabetic infertility [37].
Also, it is demonstrated that in male infertile patients, oxidative damage has a critical role in cell apoptosis [38].
Several studies reported that high concentration of glucose can induce ROS and pro-inflammatory mediators in the testis tissue in diabetic conditions which can stimulate cell death [39].
Several cellular and molecular mechanisms are proposed for cell apoptosis in diabetic subjects.
Over-expression of these mediators can induce apoptotic cell signaling in various organs. Excessive accumulation of ROS in cells leads to oxidative stress [40]. Probably, hyperglycemia initiates testis-cell apoptosis by intrinsic pathways including molecules Bcl-2, Mcl-1 and Bcl-xl. Also, other different mechanisms may trigger testicular cell death [41,42].
It seems that there is a major link between metabolic abnormalities and cell death pathways in diabetic conditions [43]. Our results showed that diabetes type 1 leads to reduced cell density and increased apoptosis in testis tissue in various time points after diabetes type 1 induction.
The probiotics used in this study lactobacillus casei and Bifidobacterium lactis are likely to reduce the level of glucose in diabetic rats by decreasing the blood glucose levels in diabetic rats, reducing the production of free oxygen radicals and reducing the oxidative stress. Possibly, by increasing the total antioxidant capacity and activating the immune system in diabetic groups Probiotic treated treatment results in a relative improvement in sperm parameters and testicular tissue.
On the other hand, due to the lack of significant changes in the expression of BAX and bcl-2 genes, it seems that the internal pathway inducing apoptosis in the studied mice is not active. It seems that apoptosis is induced through other pathways.
Given the prevalence of diabetes and the cost of treatment and its complications, according to the results of this study, regular and prolonged use of probiotics may be a complement to the main treatment for delaying the progression of diabetes and complications due to it is recommended.
It seems that products made from these probiotics can be considered as an appropriate supplement to control blood glucose.
Although the data obtained from various studies provide clear evidence of the efficacy of several probiotics in human disease, it still needs to confirm and strengthen clinical benefit.
It should be noted, however, that the mechanism of the effect of various probiotics should be described in more detail to determine the best species and probiotic species for use against a particular pathogen, and even the combination of probiotics requires more clinical research. Also, the dosage as well as the duration of treatment and the selection of the most appropriate probiotics should be considered.
The authors declared there is no conflict of interest.
Citation: Abasi S, Keshtmand Z (2021) Effect of Probiotics Bifidobacterium lactis And Lactobacillus casei on Sperm Parameters and Expression of Apoptosis BAX and Bcl2 Genes in Streptozotocin-Induced Diabetic Rats. J Prob Health. 9:235.
Received: 22-Jan-2021 Accepted: 05-Feb-2021 Published: 12-Feb-2021 , DOI: 10.35248/2329-8901.21.9.235
Copyright: © 2021 Abasi S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.