Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2019) Volume 10, Issue 2
A simple, rapid (8 min) ion chromatographic test method, using post-column electrolytic suppression, with conductivity detection, is validated for the quantification of Cl-, NO2 -, NO3 -, SO4 2- in river, dam, potable, waste water and in trade effluent, over the linear range: 1-100 mg/L for chloride, 0.05-5 mg/L for nitrite and nitrate, and 0.5-50 mg/L for sulphate. The ion chromatographic separation was carried out using a Dionex Ion PacTM AS22-fast 4 μm column (4 × 150 mm), with a flow rate of 1.2 mL/min, using Na2CO3/NaHCO3 (4.5 mM/1.4 mM) as eluent. The LOQ was in the range 0.025-0.50 mg/L, with an injection volume of 25 μL. The %RSD for retention time and peak area responses was <1%. Spiked recoveries were 87-107% for all 4 target anions. The method was suitable for routine drinking water quality assessment in line with the national regulations. The method was fully validated for accuracy, precision, recovery, LOQ, bias/trueness, selectivity, Measurement Uncertainty (MU), etc. The method validation data complied with the international requirements for testing laboratories for ISO/IEC 17025 accreditation.
Keywords: Water quality; Anions; Ion chromatography; Electrolytic suppression; Conductivity; Method validation; ISO/IEC 17025 accreditation
Umgeni Water’s (UW) core business is to treat raw water to potable standards [1], which is aligned to the World Health Organization (WHO) guide [2]. The guide [1] also stipulates that the tests must be ISO/IEC 17025 accredited [3,4]. The UW Head Office laboratory is ISO/IEC 17025-accredited to carry out the required the majority of the physico-chemical tests, e.g., turbidity, pH, anions, etc.
High levels of anions in water can be toxic. Nitrate and nitrite are of environmental importance because of their toxicity and environmental impact. Nitrate can affect the ability of oxygen transport in the human body [5]. Chloride can impart a salty/unpleasant taste to water and can cause hypertension, etc. The acceptable limits [1] for anions are: ≤ 300 mg/L for chloride (Cl-), ≤ 11 mg/L for nitrate (NO2-, ≤ 0.9 mg/L for nitrite (NO3 -), and ≤ 250 mg/L for sulphate (SO42-).
The suitability of liquid chromatography for water analysis has been proven over the last two decades. Ion chromatography (IC) is a sensitive, reliable and simple method for determining inorganic anions in various matrices. Since its introduction, [6], IC has become the preferred technique [7-15].
At the Chemistry Laboratory (UW), a Waters Ion Chromatograph (IC) (model: Waters 515)-Conductivity detector (model: Waters 432), without suppression, being fairly old (≥ 15 years) was being used to quantitate the target anions (chloride, nitrate, nitrite, sulphate) in drinking water. This test method [16] uses a large volume of organic solvent (acetonitrile); the chromatographic run time is 20 min. Samples are first separated, into “low’ and “high” range based on their conductivity; two separate calibrations were performed for quantification of the “low’ and “high” range concentrations.
The Laboratory operates 365 days a year to a Service Level Agreements (SLAs) for its tests. Reliable, analytical instruments are required to ensure continued routine service. Another important consideration is the technical simplicity that would enable laboratory technicians to use analytical equipment with minimal advanced technical skills. The market was investigated to find a suitable replacement IC, that would also address the current shortcomings.
Various techniques for the quantification of anions in drinking water and related matrices have been reported. However, they are not without limitations. The use of 2-dimensional IC has been reported, but it requires the use of 2 columns, valve switching and a gradient elution [8,11]. Whilst single injection ion-exclusion/cation-exchange chromatography has been reported for simultaneous determination of anions and cations, it required the use of 2 columns and post-column derivatization [9]. Use of ion pair chromatography with UV detection has been reported, but for saline water matrix, the mobile phase had to be modified and the elution order of the anions changed [12]. Two earlier IC-suppressed conductivity detection methods have been reported, but the elution time exceed 10 min [14,15].
The use of a suppressor device [7] provides continuous suppression of eluent conductivity and enhances analyte response, prior to entering the detector. The anions are converted to their highly conductive acid forms while the conductivity of the eluent is greatly decreased.
Whilst various IC-suppressed conductivity detection methods are provided by the manufacturer [17], the additional method validation data, to show evidence that the test is fit-for purpose, is not generally provided.
In this work, a simplified, accurate test method, by ion chromatography-suppressed conductivity detection is reported and validated, with full method validation data to meet the ISO 17025 accreditation testing requirements, for the rapid quantification of four priority target anions: chloride, nitrate, nitrite and sulphate in river, dam, potable, waste water, and in trade effluent.
Chemicals and standards
The chemicals and anion salts: sodium carbonate, sodium bicarbonate, potassium chloride, potassium nitrite, sodium nitrate, potassium sulphate, of analytical grade, and the 0.45 μm filters were purchased from Merck. Ultrapure water was obtained from a Siemens Ultra Clear water purification system (specifications: Conductivity at 25°C: 0.055 μS/cm, Resistivity at 25°C: 18.2 MΩ-cm, TOC: <1-5 ppb). Salts were dried at 105 ± 5°C for 3 hr. before use. The Sep Pak C18 cartridges were obtained from Microsep.
The aqueous Composite Stock standards for the calibration and analytical quality control (AQC) were: 1000 mg Cl-/L, 50 mg NO2- /L 50 mg NO3 -/L, 500 mg SO4 2-/L. The Composite Stock calibration solution was auto-diluted, by the equipment, to produce the following six calibration standards, of anion concentrations as follows: Standard 1: 100 mg Cl-/L, 5 mg NO2-/L, 5 mg NO3-/L, 50 mg SO4 2-/L; Standard 2: 60 mg Cl-/L, 3 mg NO2-/L, 3 mg NO3 -/L, 30 mg SO4 2-/L; Standard 3: 40 mg Cl-/L, 2 mg NO2-/L, 2 mg NO3-/L, 20 mg SO4 2-/L; Standard 4: 10 mg Cl-/L, 0.5 mg NO2-/L, 0.5 mg NO3-/L, 5 mg SO4 2-/L; Standard 5: 5 mg Cl-/L, 0.25 mg NO2-/L, 0.25 mg NO3-/L, 2.5 mg SO4 2-/L; Standard 6: 1 mg Cl-/L, 0.05 mg NO2-/L, 0.05 mg NO3 -/L, 0.5 mg SO4 2-/L. The AQC Composite Stock solution was manually diluted to give a composite working AQC of concentration: 50 mg Cl-/L, 2.5 mg NO2-/L, 2.5 mg NO3-/L, and 25 mg SO4 2-/L.
Sample collection
Umgeni Water’s Sampling Services Department is ISO 9001-accredited. All grab, water samples, from the Umgeni Water catchments (Inanda Dam, Umzinto River), from the water treatment works (Umzinto potable water works, Mpofana waste water works), and from Huletts Aluminium, Pietermaritzburg (trade effluent: Umgeni Water sample site/point WPT 002) were collected into plastic or glass bottles, as per the documented procedure. For the validation of accuracy, water samples from the South African Bureau of Standards (SABS) Proficiency testing Scheme [18], were used for chloride, nitrate and sulphate, and The ERA Water Supply for Drinking Water PT Scheme [19] was used for nitrite.
Experimental design
Our laboratory uses a standard operating procedure for method validation [20] which is fully aligned to ISO/IEC 17025, and specifically the national accrediting body South African National Accreditation Standards (SANAS) [21] document TR 26-03. TR 26-03 [22] details the typical validation parameters, like precision, accuracy, Measurement Uncertainty (MU) [23,24], etc. We use international test methods [7,25-27], technical application notes provided by the equipment manufacturer, literature reported methods, etc. The reported test method details in the Dionex IC and manufacturer operator manual were used as a guide [17,28].
Extraction procedure
Samples were filtered through 0.45 μm filters. Waste water samples were first filtered through the Sep Pak C18 cartridges, followed by filtration through the 0.45 μm filter. A 25 μL aliquot is then analyzed.
Ion chromatography conditions
The test method validation [20] and all analyses was carried out on a Dionex IC, equipped with an ICS-5000+ SP single reciprocating pump, a pull loop Dionex AS-AP auto sampler, and a Model DIONEX ICS-5000+ Detector Chromatography Module. The eluent was Na2CO3/NaHCO3 (4.5 mM/1.4 mM). Separations, at a flow rate of 1.2 mL/min, were performed on a Dionex Ion PacTM AS22-fast 4 μm column (4 × 150 mm), set to 30°C, in combination with an AG22-fast 4 μm guard column (4 × 30 mm), followed by a Dionex AERS 500 Electrolytically Regenerated carbonate suppressor, set at 20°C. The detection of the four target anions was achieved by a Dionex ICS-5000+ CD Conductivity detector, with the heater temperature set at of 35°C. The data acquisition and processing were done using the IC instrument Chromeleon Software (Version 7).
Method validation
The test method was validated using our Chemistry laboratory’s internal standard operating procedure (SOP 16b) [20]. For test method validation. Our approach is to generally focus on international test methods, like those referenced to the US EPA, the Standard Methods for the Examination of Water and Wastewater textbook, technical application notes from instrument manufacturers, etc., in order to facilitate method verification rather than a comprehensive method validation, but as per compliance to the ISO/IEC 17025 guide requirements [4,22].
Our internal protocol
Our internal SOP 16b for method validation for our laboratory [20], aligned to meeting the requirements of ISO/IEC 17025 [4,22], addresses the following parameters where applicable/practical:
Accuracy: Accuracy is the measure of how close the test results are towards the known or “true” value. Accuracy is assessed by analyzing PTS samples and calculating the Z-scores. The ideal Z-score is zero and values outside the range of +2 and -2 are not acceptable during method validation. The equation for the calculation of Z-score is given below:

Accuracy can also be assessed by analyzing a CRM and calculating the percentage error. The percentage error should be within ± 5%. The equation for the calculation of percentage error is given below:
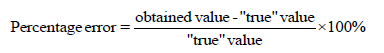
If both of the above are not possible, accuracy can be assessed by spiking a sample of real matrix and determine the recovered analyte. The acceptable percentage error should be within ± 10% error, i.e., the recovered analyte should have a concentration between 90% and 110% of the spiked concentration. The equation for the calculation of recovery is given bellow:
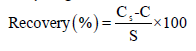
Where Cs is the spiked sample concentration, C is the sample background concentration (unspiked sample) and s is the concentration equivalent of analyte added to the sample.
Repeatability precision: This gives an idea of the sort of variability to be expected when a method is performed by a single analyst over a short time scale. This is determined by analyzing at least seven replicates of the calibration standards (at low, medium and high concentration) and calculating the %RSD. The equation for the calculation of %RSD is given below:
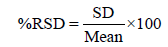
Reproducibility precision/ruggedness/robustness: This gives an idea of the sort of variability to be expected when a method is performed by a single or different analyst/s under different conditions over a long time period. This is determined by analyzing at least 40 AQC standards over a period of at least ten days. The %RSD is then computed and should be ≤ 10%.
Limit of detection (LOD): The LOD is the smallest concentration of an analyte in the test sample that can be reliably distinguished from zero concentration, however not necessarily quantified. The IUPAC method [29] is used to determine the limit of detection. This is done by analyzing a “blank’ sample at least seven times and computing the standard deviation.
LOD = Mean + 3 x Standard Deviation
If it is not possible to use the IUPAC method [29], e.g., all blanks show a zero or negative concentration, a standard is prepared with a concentration that is closer to zero but far from the lowest calibration standard and is used instead of the blank. If the prepared standard is still showing a negative or zero concentration, increase the concentration of the standard is increased, but it is still kept it far from the lowest calibration standard.
Limit of quantification (LOQ): The LOQ is the lowest concentration of an analyte in a sample that can be determined with an acceptable level of repeatability precision (% RSD ≤ 10%) and recovery (80%-120%).
The IUPAC method [29] is used to determine the limit of quantification. This is done by analyzing a blank sample at least seven times and computing the standard deviation, as follows:
LOQ = Mean + 10 x Standard Deviation
If it is not possible to use the IUPAC method [29], e.g., all blanks show a zero concentration, a standard is prepared with a concentration that is closer to zero but far from the lowest calibration standard and is used t instead of the blank. If the prepared standard is still showing a negative concentration, the concentration of the standard is increased but it is still kept far from the lowest calibration standard.
Recovery: Recoveries are measured by spiking (with low, medium and high standards) the sample matrix of interest with a known concentration of reference material. After the samples are analyzed in at least seven replicates, the results are compared with the known concentrations, and the recovery is determined. The acceptable criterion for recoveries is 80% to 120%. The percentage recoveries are computed using the following formula:
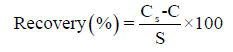
Where Cs is the spiked sample concentration, C is the sample background concentration (unspiked sample) and s is the concentration equivalent of analyte added to the sample.
Linearity: Linearity is the ability of a test method (within a specific range) to obtain test results that are directly proportional to the concentration (amount) of analyte in the sample. Linearity is determined by analysing calibration standards (at least four, including the blank) and obtaining the correlation coefficient (r2). The r2 value must be ≥ 0.995.
Sensitivity: From the regression equation for a straight line: y=mx+c, a method is calibration-sensitive if the slope (m) of the calibration graph is ≠ 0.
Working range: The working range of a test method is the interval between the lower and upper concentration of analyte in the sample for which it has been demonstrated that the test method has suitable levels of precision, accuracy and linearity. The working range is from the reporting limit to the highest standard.
Scope: The scope of a test method is demonstrated by selecting samples to cover different type of sample matrices. These samples are normally surface water (river water, lake water and dam water), sewage (wastewater), borehole (ground water) and potable water. Each sample is analyzed at least seven times and the %RSD is computed. The %RSD should be ≤ 10% for all samples; it is possible to have %RSDs >10% if the samples of interest have concentrations lower than the Quantification Limit.
Bias (systematic error): This is the tendency of a test method towards delivering a result that is skewed from the “true” value. It is the difference between the experimental mean (obtained value) and the “true” value and is generated from a total systematic error as contrasted to random error. There may be one or more systematic error components contributing to the bias. This is done in the same way as reproducibility precision/ruggedness (5.2.3). The acceptable bias should be within ± 5% and it is calculated as follows:
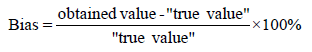
Trueness: This is the closeness of agreement between the average of an infinite number of replicate measured quantity values and a reference quantity value. Trueness is inversely related to Bias and it is calculated as follows:
Trueness = 100% + Bias
The acceptable Trueness is from 95% to 105%.
Specificity/selectivity: This is the ability of a test method to respond to a particular analyte of interest in the presence of possible interferences such as impurities, degradants and matrix effects. This is done in the same way as recovery and it has the same criteria. If the recoveries are within 80%-120%, it means that the test method is selective and specific to the analyte in the presence of real impurities and interferences.
Stability of standards: This is the measure of how long the standards can be used before they can produce unreliable test results. This must be done by preparing the standards of interest and analysing them at least once a week. If the standards still produce acceptable results, e.g., r2 ≥ 0.995, QC/PTS results are within the acceptable values, the study must be carried on up until the results are no longer within the acceptable values. The duration from when the reagent was prepared to the last week it produced reliable results will be considered as the “stability” for that reagent. This parameter might not be relevant if the historical data is available to prove the stability of standards.
Measurement uncertainty: The Measurement of uncertainty is conducted according to our laboratory internal SOP 20c [30] which is aligned to meeting the requirements of ISO/IEC 17025 [4,22].
Reporting limit: The Reporting limit is similar to the LOQ, and it must be chosen to simplify the process of reporting test results. For example, if the LOQ of a particular test method is 2.89 mg/L, the reporting limit can be chosen to be ≥ 3 mg/L. It must be noted that reporting limits must always be greater or equal to the LOQ. Also, the reporting limits must be less than or equal to the requirements of the latest SANS 241 [1] or of the General/Special Standard for Wastewater [31] or specific Customer requirements.
The test method details in the IC column product manual [17] and in the instrument manufacturer manual [28] was used as a guide for the initial IC parameters.
Column
The recommended IC column [17] was the Dionex Ion PacTM AS22-fast 4 μm column (4 × 150 mm), which was purchased at the same time as that of the IC equipment. This column was subsequently evaluated. No other stationery phases were evaluated.
Eluent
As per the recommended test method [17], the eluent used was Na2CO3/NaHCO3 (4.5 mM/1.4 mM).
Temperature
As per the recommended test method [17] the IC column temperature used was 30°C.
Injection volume
As per the recommended method [17], the injection volume used was 25 μL.
Flow rate
The column flow rate was tested at 1.0, 1.2 and 1.5 mL/min, whilst evaluating the resulting resolution of the anion peak responses and overall column pressure. We also aimed to reduce the run time to <10 min: 1.2 mL/min was found to be optimum.
The target anions were well resolved over the analytical range tested: chloride 1-100 mg/L, nitrate/nitrite: 0.05 to 5 mg/L, and sulphate: 0.5 to 50 mg/L, in the carbonate eluent used. All 4 anions eluted within 8 min: the observed retention times, at a flow rate of 1.2 mL/min, were: chloride at ± 2.80 min, nitrate at 3.29 min, nitrate at ± 4.32 min and sulphate at ± 6.86 min for an “upper/high range” standard (Figure 1).
Linear range
The calibration graphs were plotted using the standards concentration (mg/L): 1-100 mg/L for chloride, 0.05-5 mg/L for nitrate and nitrite, and 0.5-50 mg/L for sulphate, and the corresponding analyte peak area responses (uS*min units) The overlaid chromatogram at the various calibration standard concentrations is displayed in Figure 2.
The mean regression data, from 6 day-to-day calibrations, is summarized in Table 1, as mean ± SD (%RSD): When the calibration data were initially fitted to the straight line form: y = mx + c, the observed correlation coefficients (r2) were lower than 0.995 for all four anions. The regression analysis was found to be quadratic (2nd order, polynomial) of the form: y = ax2 + bx + c, where: a = C0 (Offset), b = C1 (Slope) and c = C2 (Curve). The correlation coefficient (r2) complied with the laboratory’s requirement [19] of being ≥ 0.995.
| Number | Anion | Linear range mg/L | m Mean ± SD (% RSD) | c Mean ± SD (% RSD) | r2 Mean ± SD (% RSD) |
|---|---|---|---|---|---|
| 1 | Cl- | 1-100 | 0.1254 ± 0.0127 (10.09) | - 0.0650 ± 0.0393 (60.53) | 0.9996 ± 0.0004 (0.04) |
| 2 | NO2- | 0.05-5 | 0.2211 ± 0.0222 (10.05) | - 0.0005 ± 0.0021 (313.74) | 0.9999 ± 0.0002 (0.02) |
| 3 | NO3- | 0.05-5 | 0.2400 ± 0.0303 (12.61) | - 0.0004 ± 0.0023 (- 572.06) | 0.9998 ± 0.0002 (0.02) |
| 4 | SO42- | 0.5-50 | 0.0747 ± 0.0072 (9.63) | - 0.0030 ± 0.0069 (- 229.95) | 0.9999 ± 0.0002 (0.02) |
Table 1: Typical regression data for the calibration standard graphs.
Sensitivity
Analysis of the day-to-day calibration data indicated that, for the regression equation: y = ax2 + bx + c, obtained for all four anions, the gradient (C1)b ≠ 0. It was therefore concluded that the test method is sensitive.
Precision
Instrument precision was determined by 7 replicate injections of the calibration standards: 10 mg/L for chloride, 0.5 mg/L for nitrite, 0.5 mg/L for nitrate and 5 mg/L for sulphate. The %RSD for peak response (and for retention time data) were: 0.75% for chloride (mean=2.77 min, 0.07%), 0.38 for nitrite (mean=3.31 min, 0.03%), 0.63% for nitrate (mean=4.39 min, 0.03%) and 0.37% (mean=6.95 min, 0.02%) for sulphate.
For the calibration standards (7 replicates):
At low standard concentration: 10 mg/L chloride, 0.05 mg/L nitrate and nitrite, and 5 mg/L sulphate, the observed concentration (mg/L, mean ± SD) and precision (% RSD) was: 10.19 ± 0.04 (0.37%) for chloride, 0.56 ± 0.00 (0.43%) for nitrite, 0.57 ± 0.00 (0.61%) for nitrate and 5.51 ± 0.02 (0.38%) for sulphate.
At medium standard concentration: 50 mg/L chloride, 2.5 mg/L nitrate and nitrite, and 25 mg/L sulphate, the observed concentration (mg/L, mean ± SD) and precision (%RSD) was: 52.07 ± 0.22 (0.43%) for chloride, 2.61 ± 0.01 (0.36%) for nitrite, 2.59 ± 0.01 (0.41%) for nitrate and 25.88 ± 0.11 (0.43%) for sulphate.
At high standard concentration: 80 mg/L chloride, 4 mg/L nitrate and nitrite, and 40 mg/L sulphate, the observed concentration (mg/L, mean ± SD) and precision (%RSD) was: 80.73 ± 0.32 (0.39%) for chloride, 4.05 ± 0.01 (0.35%) for nitrite, 4.02 ± 0.02 (0.40%) for nitrate and 40.19 ± 0.16 (0.39%) for sulphate.
In addition, for the analysis of the real matrix samples, the observed precision (%RSD), based on 7 replicates, is summarized in Table 4 : 0.39- 1.86% for chloride, 0-0.69% for nitrate and 0.36-0.99% for sulphate; the nitrite concentration ranged from “not detected” to
Reproducibility precision/ruggedness/robustness/intermediate precision
The AQC samples: 50 mg/L chloride, 2.5 mg/L nitrate and nitrite, 25 mg/L sulphate, (n=40), were analyzed over the period: 02/08/2018 to 15/12/208: The observed mean concentrations (mg/L) (± SD, and %RSD) were: 51.43 (0.90, 1.74%) for chloride, 2.55 mg/L (0.04, 1.69%) for nitrate, 2.59 (0.07, 2.73%) for nitrite and 25.49 (0.41, 1.59%) for sulphate.
Sensitivity: LOD and LOQ
It was not practical to determine numerical values for the LOD: replicate analysis of a blank ultrapure water sample (Figure 3) gave a “n.a.” (not applicable) output on the instrument software.
Figure 4 shows the chromatogram of the lowest anion standard: 0.25 mg/L for chloride, 0.0125 for nitrate and nitrite, and 0.125 mg/L for sulphate, that gave a peak response.
For the LOQ, we use a criterion of 80-120% recovery and ≤ 10% RSD that can be achievable, for the lowest aqueous standard concentration, from 10 replicate injections of the lower range standard, which is serially diluted and is then analyzed as a sample. The LOQ was found to be: 0.50 mg/L for chloride, 0.025 mg/L for nitrate and nitrite, and 0.25 mg/L for sulphate (Figure 5); the observed recovery was 88- 100% (RSD=3.12-4.70%) at these standard concentrations.
Scope
The sample scope included the analysis of river, dam, drinking, domestic waste water and trade effluent matrices (Table 4).
Recovery
Matrix samples were spiked with the “low, medium and high” calibration standards: with 10, 50 and 80 mg/L for chloride, with 5, 25 and 40 mg/L for sulphate, and with 0.5, 2.5 and 4 mg/L for nitrate and nitrite. All spiked samples were analyzed in 10 replicates. The recovery data are summarized in Table 2.
| Matrix | Spike level | % Recovery | |||
|---|---|---|---|---|---|
| Cl- | NO2- | NO3- | SO42- | ||
| Dam: Inanda Dam | 1 “low” | 94.70 | 95.93 | 92.01 | 92.25 |
| 2 “medium” | 96.29 | 100.30 | 95.77 | 95.88 | |
| 3 “high” | 93.80 | 100.20 | 95.20 | 94.50 | |
| m | 0.1946 | 0.3051 | 0.3403 | 0.1074 | |
| c | - 0.1568 | - 0.0026 | - 0.0018 | - 0.0111 | |
| r2 | 0.9997 | 0.99996 | 1.0000 | 0.9999 | |
| Mean | 94.93 | 98.81 | 94.33 | 94.21 | |
| SD | 1.26 | 2.50 | 2.03 | 1.83 | |
| % RSD | 1.33 | 2.53 | 2.15 | 1.94 | |
| Potable: Umzinto potable water works | 1 “low” | 89.81 | 87.27 | 101.80 | 89.88 |
| 2 “medium” | 95.26 | 106.45 | 102.35 | 99.72 | |
| 3 “high” | 90.50 | 106.60 | 98.80 | 96.40 | |
| m | 0.1896 | 0.2743 | 0.3355 | 0.1056 | |
| c | - 0.1398 | - 0.0007 | - 0.0011 | - 0.0070 | |
| r2 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | |
| Mean | 91.86 | 100.11 | 100.98 | 95.33 | |
| SD | 2.97 | 11.12 | 1.91 | 5.01 | |
| % RSD | 3.23 | 11.11 | 1.89 | 5.25 | |
| River: Umzinto River | 1 “low” | 94.04 | 103.70 | 102.66 | 98.25 |
| 2 “medium” | 93.97 | 103.81 | 101.74 | 99.30 | |
| 3 “high” | 88.70 | 99.90 | 97.60 | 95.00 | |
| m | 0.1862 | 0.2939 | 0.3246 | 0.1023 | |
| c | - 0.1251 | 0.0004 | 0.0010 | - 0.0013 | |
| r2 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | |
| Mean | 92.24 | 102.47 | 100.67 | 97.52 | |
| SD | 3.06 | 2.23 | 2.70 | 2.24 | |
| % RSD | 3.32 | 2.17 | 2.68 | 2.30 | |
| Waste: Mpofona Waste Water Works | 1 “low” | 102.02 | 101.90 | 101.80 | 98.23 |
| 2 “medium” | 101.75 | 105.08 | 102.35 | 100.63 | |
| 3 “high” | 98.80 | 104.30 | 98.80 | 99.50 | |
| m | 0.1036 | 0.1885 | 0.1990 | 0.0634 | |
| c | - 0.0534 | 0.0005 | 0.0004 | 0.0011 | |
| r2 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | |
| Mean | 100.86 | 103.76 | 100.98 | 99.45 | |
| SD | 1.79 | 1.66 | 1.91 | 1.20 | |
| % RSD | 1.77 | 1.60 | 1.89 | 1.21 | |
| Trade effluent: Huletts Aluminium Ltd (WPT002) |
1 “low” | 90.34 | 100.08 | 104.37 | 88.63 |
| 2 “medium” | 105.90 | 104.55 | 106.31 | 95.50 | |
| 3 “high” | 102.30 | 100.60 | 102.60 | 92.50 | |
| m | 0.1109 | 0.1985 | 0.2111 | 0.0671 | |
| c | - 0.0606 | 0.0005 | 0.0005 | 0.0008 | |
| r2 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | |
| Mean | 99.51 | 101.74 | 104.43 | 92.21 | |
| SD | 8.14 | 2.45 | 1.86 | 3.45 | |
| % RSD | 8.18 | 2.41 | 1.78 | 3.74 | |
Table 2: Recovery data for the anions from spiked matrix samples.
The recovery, for all anions, ranged from 87-107%, with ≤ 10% RSD, except for NO2 -(%RSD=11%) for the Umzinto drinking water sample.
Accuracy
The external PTS z-scores (Table 3), all within the acceptable range: -2
| Matrix | Spike level | % Recovery | |||
|---|---|---|---|---|---|
| Cl- | NO2- | NO3- | SO42- | ||
| Dam: Inanda Dam | 1 “low” | 94.70 | 95.93 | 92.01 | 92.25 |
| 2 “medium” | 96.29 | 100.30 | 95.77 | 95.88 | |
| 3 “high” | 93.80 | 100.20 | 95.20 | 94.50 | |
| m | 0.1946 | 0.3051 | 0.3403 | 0.1074 | |
| c | - 0.1568 | - 0.0026 | - 0.0018 | - 0.0111 | |
| r2 | 0.9997 | 0.99996 | 1.0000 | 0.9999 | |
| Mean | 94.93 | 98.81 | 94.33 | 94.21 | |
| SD | 1.26 | 2.50 | 2.03 | 1.83 | |
| % RSD | 1.33 | 2.53 | 2.15 | 1.94 | |
| Potable: Umzinto potable water works | 1 “low” | 89.81 | 87.27 | 101.80 | 89.88 |
| 2 “medium” | 95.26 | 106.45 | 102.35 | 99.72 | |
| 3 “high” | 90.50 | 106.60 | 98.80 | 96.40 | |
| m | 0.1896 | 0.2743 | 0.3355 | 0.1056 | |
| c | - 0.1398 | - 0.0007 | - 0.0011 | - 0.0070 | |
| r2 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | |
| Mean | 91.86 | 100.11 | 100.98 | 95.33 | |
| SD | 2.97 | 11.12 | 1.91 | 5.01 | |
| % RSD | 3.23 | 11.11 | 1.89 | 5.25 | |
| River: Umzinto River | 1 “low” | 94.04 | 103.70 | 102.66 | 98.25 |
| 2 “medium” | 93.97 | 103.81 | 101.74 | 99.30 | |
| 3 “high” | 88.70 | 99.90 | 97.60 | 95.00 | |
| m | 0.1862 | 0.2939 | 0.3246 | 0.1023 | |
| c | - 0.1251 | 0.0004 | 0.0010 | - 0.0013 | |
| r2 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | |
| Mean | 92.24 | 102.47 | 100.67 | 97.52 | |
| SD | 3.06 | 2.23 | 2.70 | 2.24 | |
| % RSD | 3.32 | 2.17 | 2.68 | 2.30 | |
| Waste: Mpofona Waste Water Works | 1 “low” | 102.02 | 101.90 | 101.80 | 98.23 |
| 2 “medium” | 101.75 | 105.08 | 102.35 | 100.63 | |
| 3 “high” | 98.80 | 104.30 | 98.80 | 99.50 | |
| m | 0.1036 | 0.1885 | 0.1990 | 0.0634 | |
| c | - 0.0534 | 0.0005 | 0.0004 | 0.0011 | |
| r2 | 0.9999 | 1.0000 | 1.0000 | 1.0000 | |
| Mean | 100.86 | 103.76 | 100.98 | 99.45 | |
| SD | 1.79 | 1.66 | 1.91 | 1.20 | |
| % RSD | 1.77 | 1.60 | 1.89 | 1.21 | |
| Trade effluent: Huletts Aluminium Ltd (WPT002) |
1 “low” | 90.34 | 100.08 | 104.37 | 88.63 |
| 2 “medium” | 105.90 | 104.55 | 106.31 | 95.50 | |
| 3 “high” | 102.30 | 100.60 | 102.60 | 92.50 | |
| m | 0.1109 | 0.1985 | 0.2111 | 0.0671 | |
| c | - 0.0606 | 0.0005 | 0.0005 | 0.0008 | |
| r2 | 0.9997 | 0.9999 | 0.9999 | 0.9999 | |
| Mean | 99.51 | 101.74 | 104.43 | 92.21 | |
| SD | 8.14 | 2.45 | 1.86 | 3.45 | |
| % RSD | 8.18 | 2.41 | 1.78 | 3.74 | |
Table 3: External accuracy assessment by analysis of PTS samples.
Bias and trueness
Using the observed recovery data, the calculated mean Bias values (range) were: +4.12 (-11.3 to +5.90) for chloride, -1.38 (-12.3 to +6.60) for nitrite, -0.28 (-7.99 to +6.3)1 for nitrate and +4.26 (-11.47 to +0.63) for sulfate.
Specificity/selectivity
A low range anion composite standard was spiked, separately, with three interferents at a concentration of 10 mg/L: fluoride (Figure 6), bromide (Figure 7) and phosphate (Figure 8) standard; all spiked samples were analyzed in 10 replicates:
All interferent peaks were well resolved from the 4 analyte peaks of interest. The observed anion recoveries were: 83-96% for chloride, 96-102% for nitrite, 90-102% for nitrate and for sulfate.
MU
The maximum expanded Measurement Uncertainty (MU) [23,24] was found to be <10%: UOM= ± 6.3% for chloride, ± 6.3% for nitrite, ± 6.7% for nitrate, and ± 4.7% for sulfate. As per the requirements of our internal SOP 16c, these values are within 10%.
Reporting Limit
The Reporting limit is similar to the LOQ. The LOQ was 0.50 mg/L for chloride, 0.025 mg/L for nitrate, 0.025 mg/L for nitrite and 0.25 mg/L for sulphate. The reporting limits were chosen to be: 1 mg/L for chloride, 0.1 mg/L for nitrate, 0.1 mg/L for nitrite and 1 mg/L for sulphate. This was done to simplify the process of reporting test results.
Application-analysis of real samples for anions
Real samples were analyzed by the developed method. The observed data, for 10 replicate analyses, is summarized in Table 4.
| Water sample type/matrix | Anion concentration Mean ± SD (mg/L) (% RSD) | |||
| Cl- | NO2- | NO3- | SO42- | |
| Dam: Inanda Dam |
34.56 ± 0.20 (0.58) |
< LOQ 0.011 ± 0.004 (33.07) |
1.39 ± 0.01 (0.69) |
16.29 ± 0.11 (0.66) |
| River: Umzinto River |
44.39 ± 0.22 (0.49) |
< LOQ 0.004 ± 0.001 (31.75) |
0.30 ± 0.01 (1.81) |
10.49 ± 0.04 (0.36) |
| Potable: Umzinto Potable Water Works |
48.83 ± 0.19 (0.39) |
ND* | 0.67 ± 0.00 (0.00) |
11.70 ± 0.06 (0.49) |
| Waste: Mpofana Waste Water works | 35.53 ± 0.21 (0.60) |
ND | ND | 16.00 ± 0.08 (0.51) |
| Trade effluent: WPT002 |
1.29 ± 0.02 (1.86) |
ND | ND | 26.18 ± 0.26 (0.99) |
Table 4: Real matrix samples analysis for the target anions.
The developed test method is suitable, sensitive, precise and accurate for the various sample types, for the quantification of the anions over the concentration range: 0.5-100 mg/L for chloride, 0.025-5 mg/L for nitrate and nitrite, and 0.25-50 mg/L for sulphate. The observed concentrations for the anions in potable water were complaint with the limits for drinking water as per SANS 241: ≤ 300 mg/L for chloride (Cl- ), ≤ 11 mg/L for nitrate (NO2 -, ≤ 0.9 mg/L for nitrite (NO3 -), and ≤ 250 mg/L for sulphate (SO4 2-).
Comparison with other reported IC methods
In 1993, the US EPA advocated suppressed ion chromatography for anions analysis in drinking water [7]. Subsequently, various ICsuppressed conductivity test methods have been reported (Table 5).
The various hardware components, and the software, of this new IC, contribute to overall improved productivity, expanded capabilities and improved performance [33]. The Dionex Ion PacTM AS22-Fast-4 μm column (4 × 150 mm)) resin composition is a supermacroporous polyvinyl benzyl ammonium polymer cross-linked with divinylbenzene. The selectivity of the Dionex IonPac AS22-Fast-4 μm column has been designed to retain fluoride well out of the water dip (system dip) and to isocratically separate common anions including carbonate [17].
The referenced test method using carbonate/bicarbonate as eluent [34] was not available at the time of this research. Whilst there are 2 additional Thermo Fisher application notes, with carbonate/bicarbonate buffer as eluent [35,36], the total run time is over 8 min [35,36]. Critical method validation performance data, like LOQ, external accuracy performance by proficiency testing [37], inter-laboratory testing [37], a mandatory requirement for ISO/IEC accredited testing laboratories [4] and UOM values are not generally reported (Table 5). Many researchers tend to evaluate recovery and use this parameter alone for assessing accuracy.
| Method/Detection | Sample | Sample pre-treatment | Analytes | Precision Within-day RSD % |
Precision Day-to-day RSD % |
LOD | LOQ | Recovery % |
Linear range | Biasa % |
External Accuracy assessment |
Reference |
| IC, Conductivity, Suppression |
Reagent water, drinking water, surface water, mixed domestic and industrial wastewater, ground water, solid/shale | Direct injection |
Br-, Cl-, F-, NO3-, NO2-, ortho-PO43-, SO42- (and Bromate, chlorate, chlorite) |
NRb | NR | MDLc 0.004-0.02 mg/L (0.003-0.02 mg/L) |
NR | 82-121% (88-155) | 0.26-95.0 mg/L | Cl- -51.0 to +7.7 NO2- -16.7 to +6.0 NO3- -28.6 to +9.5 SO42- -60.7 to +1.1 |
NR | [7] |
| IC, Conductivity, Chemical suppression |
Potable, Non-drinking |
Cl-, NO3-, SO42- | = 10% | = 10% | 0.13-0.22 mg/L | 0.45-0.72 mg/L | NR | 0.5-300 mg/L | NR | NR | [15] | |
| IC Conductivity, Suppressed IC, |
Power plant Water-steam | Filtration through 0.2 µm filter | F-, Cl-, NO3-, PO43-, SO42-, | 0.8-8% (retention time and peak area) | NR | 0.077-0.200 µg/L | NR | 60-120% | NR | -40 to +20 | NR | [14] |
| Column switching IC, conductivity, suppression | Sea water | Diluted 5-fold | F-, NO2-, NO3-, Br-, SO42-, PO43- | < 4% | NR | 2-23 µg/L | NR | 97-101% | 0.05-25 mg/L | -3 to+1 | NR | [13] |
| Ion pair reversed phase LC UV |
Potable water, Saline water, Sea water |
NO2-,NO3-, Br-, BrO3- | NR | NR | 0.20-0.60 mg/L | NR | 75-85 | 0.3-20 | -25 to -15 | NR | [12] | |
| 2D-IC, Conductivity Suppression |
Mineral water | Anions Cl-, SO42-, NO2-, NO3-, F-, Br-, PO43-, (Cations: Na+, NH4+, K+, Mg2+,Ca2+ ) |
0.60-3.0% | 0.78-3.1% | 0.001-0.3 mg/L | NR | 88.7-110% | 0.020-50.0 mg/L | -11.3 to +10 | NR | [11] | |
| Conductivity, suppression | Orange, Apple juice |
Diluted 50 and 100 times | Inorganic anions: NO3-, Cl-, SO42- organic acids | 0.3-2.6% | 0.7-5.7% | 0.03-0.8 mg/L | NR | NR | 0.1-200 mg/L | NR | NR | [10] |
| Ion exclusion-chromtography-2D IC Conductivity suppression |
Weak acids | 10-fold dilution with deionised water | Cl-, Br-, NO3-, HPO42- | < 1.89% (retention time, peak area) | NR | 2.1-32.6 ng/L | NR | 84.6-105.6% | 0.1-100 µg/L | -15.4 to +5.6 | NR | [8] |
| IEC-cation exchange chromatography, | Beer samples | Organic/ inorganic anions, (Inorganic cations; Ethanol) |
NR | NR | NR | NR | NR | NR | NR | NR | [9] | |
| IC, gradient conductivity, suppression |
Carbonated beverage | Cl-, NO3-, SO42-, PO43-, citrate |
NR | NR | NR | NR | NR | 1-50 mg/L | NR | NR | [32] | |
| IC Conductivity Suppression |
Surface, ground, waste, drinking | Filtration through 0.45 µm | F-, Br-, PO43- Cl-,NO2-, NO3-, SO42- |
NR | 2.45-7.92 | 2.0-18 µg/Lc | NR | 93-109 | NR | -7 to +9 | NR | [33] |
| IC Conductivity Suppression |
Surface, drinking, waste, trade effluent | Filtration through 0.45 µm | Cl-, NO2-, NO3-, SO42- |
0.58-0.55 | 1.59-2.73 | Cl- 0.1 mg/L, NO2-,0.008 mg/L, NO3- 0.008 mg/L, SO42- 0.083mg/Ld |
0.025-0.44 mg/L | 87.27-106.60 | 0.05-100 mg/L | -12.73 to +6.60 | PTS Z-scores within +2 to -2 |
This reported method |
a Calculated from observed recovery in absence of reported data
b not reported
c Method Detection Level
d calculated, based on validated LOQ and IUPAC rules.
Table 5: Comparison of some IC test methods for anions analysis.
The MDL is defined as 3.14 SD for n=7 replicates [29,38]. Similarly, the IUPAC defines the LOD as 3 SD, and the LOQ as 10 SD [29]. When the LOD is calculated as 3.3 SD, the ratio between LOQ and LOD is a factor of 3. Application of the IUPAC recommendation to our LOQ data gives the calculated LOD values: 0.17 mg/L (0.5/3) for chloride, 0.008 mg/L (0.025/3) for nitrite and nitrate, and 0.083 mg/L (0.25/3) for sulphate. Practically, we were able to detect 0.25 mg/L for chloride, 0.0125 for nitrate and nitrite, and 0.125 mg/L for sulphate (Figure 4).
Thus, our test method has the following major advantages: it is rapid (8 min run time); it has been validated for accuracy, LOQ and Measurement; it has been thoroughly validated, and has been found to meet testing requirements for ISO/IEC 17025 accreditation. We have established that its overall performance confirms the initially reported references [17,34]. Internally, we were successful in addressing some of the major disadvantages of our previous test method [16].
An ion chromatographic test method for low level quantification of chloride, nitrate, nitrite and sulphate, in various water matrices, was fully validated, as per ISO 17025 accreditation requirements. The method was found to be rapid, sensitive, accurate, precise and selective for routine quantification of the key anions in drinking water as per the national water quality regulations. The test method will be submitted to SANAS shortly for audit assessment for conferring of testing compliance to ISO/IEC 17025 accreditation standards.
The authors are grateful to Umgeni Water, Treasury, for the Capital Expenditure (CAPEX) funding of the IC capital equipment.
None.