Journal of Chromatography & Separation Techniques
Open Access
ISSN: 2157-7064
ISSN: 2157-7064
Research Article - (2015) Volume 6, Issue 7
In this work the potential use of powder corn cobs (PCC) for the removal of Mn (Ð) from aqueous solutions has been investigated. Experiment results indicate that the uptake of Mn (II) decreased with increasing temperature. The adsorption kinetic data indicate that the process was well described by a pseudo-second order model. Thermodynamic parameter data indicate that the process is exothermic in nature and the values of ΔH° and activation energy confirmed that Mn (II) adsorption by PCC could be attributed to a physical adsorption process.
Keywords: Adsorption; Powder corn cobs; Kinetic; Thermodynamic; Activation energy
Water in the ground is the major source of drinking water in the Middle East. Manganese ion is common contaminants in groundwater. The standard value of manganese ions in drinking has set as 0.05 mg/L according the World Health Organization (WHO) [1]. The presence of high concentrations of Mn (II) in liquid phase leads to economic and technological problems. Manganese is toxic to the brain, resulting in neurological disorder similar to Parkinson’s disease [2]. Various processes are used for removing heavy metals from water and wastewater. These processes include: chemical precipitation [3], coagulation/ flocculation [4,5], ion exchange/ solvent extraction [6,7], cementation [8], electrochemical operation [9], biological operations [10], evaporation [11], filtration [12] and adsorption [13]. Most of these processes have some disadvantages such as high capital and operational cost, requirements of expensive equipment and incomplete metal removal. Adsorption is one of the effective processes for heavy metals removal from water and wastewater Activated carbon is the famous adsorbent materials because of high sorption capacity but also high cost. Low cost, availability and highly effective adsorbents are still needed in this technology. The objective of this work is to investigate the removal of Mn (II) ions from aqueous solution using powder corn cobs (PCC) in batch technique. The kinetics of the process at different temperature are investigated using pseudo-first order and pseudosecond order kinetics models. Thermodynamic parameters and activation energy are also studied.
Preparation of adsorbent material
Powder corn cobs (PCC) were washed with distilled water to remove dirt materials. Then it is dried in an oven at 80°C. The dried PCC was ground and sieved to obtain particle size of 300-425 μm and stored in dissector for further use. The adsorbent material was characterized by zeta potential, surface areas and pore volume and EDX analysis.
Preparation of Mn (II) stock solution
Stock solutions containing 1000 mg/l of Mn (II) were prepared by dissolving appropriate amounts of MnCl2 in distilled water. For experiments, the required concentrations were prepared by dilution. The Mn (II) concentrations were determined by atomic adsorption spectrophotometer (Perkin Elmer, A 800). All chemicals and reagents used in this work were analytical grade.
Adsorption experiments
Batch technique was carried out to determine the adsorption potential of PCC to Mn (II) from aqueous solution and to investigate the optimum kinetics and thermodynamic parameters of the sorption process by shaking 50 ml of a certain concentration of Mn (II) solution and a certain amount of PCC in a thermostatic shaker water bath at 200 RPM for a desirable time, temperature and initial pH. The suspensions were withdrawn and centrifuged at 5000 rpm for 5 min and the supernatant solutions were analyzed. The pH values of suspensions were adjusted with dilute HCl or NaOH solution.
Adsorption capacity (q) of Mn (II) was defined as:
q = (C0 −Ce )V / M (1)
The removal efficiency (Re) is calculated according to the following equation:
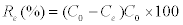 (2)
(2)
Where C0 and Ce are the concentrations of Mn (II) at initial and equilibrium time, respectively, V is the volume of the solution (L) and M is the mass of dry adsorbent used (g).
Characteristics of adsorbent
PCC was characterized by zeta potential, surface areas and pore volume and EDX analysis. The value of pHpzc was found to be 6.5. The BET surface area and pore volume of the PCC were found to be 4.325 m2/g, 0.0098 cm3/g respectively. The purpose of EDX analysis was carried out to determine the elemental composition in the PCC. The EDX spectra of the PCC before and after adsorption of Mn (II) are shown in Figure 1. It was found that carbon and oxygen were the major constituents. The feature of Mn (II) was observed in EDX spectrum of PCC after adsorption.
Adsorption dynamics
The effect of contact time is an important parameter in determining the equilibrium time that should be allowed for the maximum uptake of an adsorptive by a given adsorbent. Meanwhile, the determination of the uptake from an adsorptive at different time intervals permits the construction of an adsorption kinetic curve from which the adsorption rate constant and the order of the adsorption process could be obtained. Figure 2a shows that the removal of Mn (II) by PCC was found to be increased with increasing the time and attained a maximum value at 60 min. The removal of heavy metal ions from aqueous solution is known to be strongly dependent on the pH value of the solution [14]. The solution pH affects the surface charge on the solid particles and the solubility of the metal ions. The value of pHpzc was found to be 6.5 as mentioned above, indicating that the PCC surface carries some positive-charge functional groups. As a result, only anionic species could be adsorbed onto the adsorbent surface below its pHpzc value. The decreasing in the removal percent at lower pH is due to the competition between H+ and Mn (II) ions. However, with increasing pH the removal percent increased due to the decreasing in H+ ions that provided more adsorption sites on the PCC surface. The optimum pH for Mn (II) uptake by PCC was at pH 5 (Figure 2b). On changing the temperature from 301 to 333 K, the adsorption capacity of Mn (II) decreased from 19.34 mg/g to 6.23 mg/g (Figure 2a). The decreasing in the adsorption capacity with increasing in temperature indicating that the adsorption of Mn (II) by PCC was exothermic in nature [15]. The decreasing in the adsorption capacity of PCC with increasing the temperature may be attributed to the relative increase in the escaping tendency of the Mn (II) from the solid phase to the bulk phase or due to the weakness of adsorptive forces between the active sites of the adsorbents and the adsorbate species [16]. On changing the initial concentration of Mn (II) solution from 10 to 50 mg/l, the removal efficiency of Mn (II) decreased (Figure 3a). The uptake of Mn (II) was studied using different doses of PCC (0.3, 0.6, 0.9, 1.2, 1.5 g). The results indicated that the percent of adsorption increased with increase PCC dose due to the increasing of sorption sites (Figure 3b).
Kinetics studies
Adsorption of Mn (II) ions is analyzed using the Lagergren-firstorder and pseudo-second order kinetic equations. The Lagergren-firstorder model describes the sorption capacity of solids in solid–liquid systems [17]. It is supposed that one adsorbate is adsorbed onto one sorption site on adsorbent surface. The linear form of pseudo first order model was given by equation:
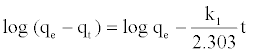 (3)
(3)
Where qe is the amount of Mn (II) adsorbed onto PCC at equilibrium (mg/g), qt (mg/g) is the amount of Mn (II) adsorbed onto PCC at time t (min) and k1 is the sorption rate constants of pseudofirst- order model (1/min). Values of k1 and qe were calculated from the slope and intercept values of the straight line of plotting log (qe-qt) versus t, respectively (Figure 4a). Pseudo-second-order model has been applied for the analysis of kinetics from liquid solutions. The linear form of pseudo-second order model [18] given by the equation:
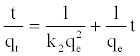 (4)
(4)
Where k2 is the sorption rate constants of pseudo-second-order (g/mg/min). The plot of t/qt versus t should give a straight line and the K2 and qe were calculated from the values of intercept and slope, respectively (Figure 4b). Figure 4 shows a fit of the sorption kinetics of Mn (II) at different temperature. The values of adsorption rate constant and the correlation coefficient are listed in Table 1. The results indicated that the pseudo-second-order kinetic model gave a good fit to the data for adsorption of Mn (II) onto the PCC.
| Parameter | Pseudo first-order (Lagergren) | Pseudo second-order | |||||
|---|---|---|---|---|---|---|---|
| K1 | qe | r/2 | K2 | qe | r2 | ||
| Temperature (K) (Condition: 100 mg L-1, 60 min, Mass=0.3 g, pH=5) | 303 | 0.0105 | 13.32 | 0.922 | 0.0104 | 19.99 | 0.998 |
| 313 | 0.0089 | 13.02 | 0.901 | 0.0068 | 18.33 | 0.996 | |
| 323 | 0.0026 | 10.32 | 0.938 | 0.0048 | 16.32 | 0.999 | |
| 333 | 0.0022 | 9.125 | 0.923 | 0.0034 | 13.01 | 0.995 | |
Table 1: Kinetics data for adsorption of Mn (II) onto PCC.
Thermodynamic studies
Gibbs free energy (ΔGº) enthalpy (ΔHº) and entropy (ΔSº) are important thermodynamic parameters. The parameters were calculated from the curve relating to the equilibrium constants (Kc) as a function of temperature using the equation [15,16]
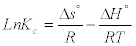 (5)
(5)
Where, R is the gas constant (8.314 J/mol K) and T is the temperature (K). The equilibrium constants Kc was calculated at each temperature using the following relationship:
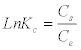 (6)
(6)
Where, Cs is the Mn (II) adsorbed on solid phase at equilibrium and Ce is the equilibrium concentration in solution.
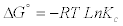 (7)
(7)
The values of ΔH°, ΔS° were determined from the slope and intercept values of the straight line of plotting lnKc versus 1/T(K), respectively (Figure 5a). From Table 2, the negative value of ΔH°, indicating that the adsorption process is exothermic in nature. Also, the value of ΔH° may give an indication about the type of adsorption of Mn (II) ions onto PCC. In the physical adsorption ΔH° falls into a range 2.1–20.9 kJ/mol, while in the chemisorption generally falls into a range of 80- 200 kJ/mol [19]. Table 2 shows that the value of ΔH° is (-17.27 kJ/ mol) indicates that Mn (II) adsorption by PCC could be attributed to a physical adsorption process. The negative value of ΔGo indicates the feasibility of the process and the spontaneous in nature. The negative value of ΔS° indicates a decrease in randomness at the solid/solution interface during the adsorption of Mn (II) ions onto PCC.
| ΔH° (kJ/mol) | ΔS° (KJ/mol.K) | ΔG°(kJ/mol) | |||
|---|---|---|---|---|---|
| 303 K | 313 K | 323 K | 333 K | ||
| -17.29 | -0.0502 | -0.0025 | -0.00243 | -0.0013 | -0.0012 |
Table 2: Thermodynamic parameters of adsorption of Mn (II) onto PCC at different temperatures.
Activation energy
In order to further support the assertion that physical adsorption is the predominant mechanism, the values of activation energy (Ea) was estimated from the experimental data. The magnitude of activation energy gives an indication about the type of adsorption, which is physical or chemical adsorption. The physisorption processes usually have activation energies in the range of 0-40 kJ/mol, while higher activation energies (40-800 kJ/mol) suggest chemisorption [20]. The decreasing in the rate constant of pseudo-second order model with temperature may be described by the Arrhenius equation which is used to calculate the activation energy, as shown by following equation [21]
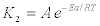 (8)
(8)
Where, k2 is the rate constant of pseudo-second order model, Ea is the activation energy (kJ/mol), A the Arrhenius factor, R the gas constant (8.314 J/mol K) and T is the absolute temperature (K). The linear form of Arrhenius equation, given by the equation:
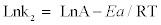 (9)
(9)
When ln k2 is plotted versus 1/T, a straight line with slope –Ea/R is obtained (Figure 5b). The obtained value of activation energy (27.37 kJ/mol) confirms that the adsorption of Mn (II) onto PCC is physisorption.
The results obtained in this study clearly demonstrated the potential use of PCC for the removal of Mn (II) from aqueous solutions. The experimental data, suggesting that the adsorption process tends to follow the pseudo-second order kinetic model. Thermodynamic parameters indicated that the adsorption of Mn (II) onto PCC was exothermic in nature. The magnitude values of ΔH° and activation energy indicate that Mn (II) adsorption by PCC could be attributed to a physical adsorption process.