Journal of Clinical and Experimental Ophthalmology
Open Access
ISSN: 2155-9570
ISSN: 2155-9570
Review Article - (2023)Volume 14, Issue 4
Ocular angiogenesis is an important contributor to many vision-decreasing diseases, such as retinopathy of prematurity, proliferative diabetic retinopathy, as well as age-related macular degeneration. Understanding the molecular mechanism of angiogenesis has advanced the development of effective medicine in preventing ocular neovascularization. Phosphoinositide 3 kinases (PI3Ks), a main downstream of growth factors, are a family of lipases that are evolutionarily conserved in their ability to regulate cell growth and survival as well as migration and metabolism. Much evidence showed that it also plays a significant role in angiogenesis. Here, we review the distinct functions of class I PI3Ks in the canonical angiogenic pathway and its potential mechanism as a novel therapeutic target in ocular pathological angiogenesis.
Isoform; Lipases; Ocular angiogenesis, photoreceptors; Vascular endothelial growth factor
Ocular angiogenesis, which is characterized by the immaturity of new blood vessels in the eye, underlies the leading causes of blindness across different age groups [1]. Current understanding of these conditions involved growth factors mainly refer to Vascular Endothelial Growth Factor (VEGF), and limited therapeutic options present variable efficacy [2]. Thus, researchers have continued to investigate the molecular mechanisms that underlie ocular angiogenesis in recent decades, and the PI3K pathway has been shown to have a role in the promotion of ocular angiogenesis.
At present, Bevacizumab, Razumab, Abercept, and Compacept against VEGF have been widely applied in ophthalmic diseases. Anti-VEGF medications can restrict the formation of new blood vessels and reduce vascular leakage, nevertheless, resistance to these drugs as well as disease recurrence has been found in a large number of patients with vascular disease [3]. In addition, considering the important neuroprotective effect of VEGF, long- term anti-VEGF therapy’s possible adverse effects on Muller cells, photoreceptors, and retinal cone cells also limit its application, and the search for new therapeutic approaches is imminent [4-6].
PI3Ks are a family of lipid kinases that regulate cellular activities through signaling downstream of a variety of cell surface receptors [7]. A total of three classes of PI3Ks have been identified. Class I PI3Ks, most clearly studied, are involved in signaling downstream of plasma membrane-bound receptors and small GTPases, whereas class II and class III PI3Ks are primarily involved in membrane transport and primarily regulate signaling indirectly, respectively [8]. It is generally accepted that class I PI3K plays a principal role in the angiogenesis, and increasing evidence suggests that class II and III PI3K also contribute to vessel growth, here we mainly focus on how class I might become a new therapeutic target for ocular neovascular diseases [9-13]. This article reviews the distinct functions of class I PI3K isoforms in the canonical angiogenic pathway and its corresponding mechanism as a novel therapeutic target in ocular pathological angiogenesis.
The process of angiogenic vessel growth
General process of angiogenesis and main factors: Angiogenesis refers to the proliferation and migration of vascular endothelial cells based on the original capillary, resulting in the formation of new capillaries from the existing blood vessels in the form of sprouting or non-sprouting (commonly known as intussusception).
The approximate steps in angiogenesis have been elucidated in Figure 1. It is generally divided into vascular expansion and increased permeability, with inflammatory cell infiltration; degradation of extravascular matrix and basement membrane by Matrix Metalloproteinase (MMP); activation, proliferation, and migration of vascular endothelial cells, accompanied by vascular germination, lumen formation, and vascular anastomosis and vascular maturation based on pericyte maturation [14-16]. In the process of neovascularization, hypoxia is the initial inducing factor leading to the upregulation of some growth factors, integrins, and proteases, which promotes the proliferation and migration of vascular endothelial cells (Figure 1).
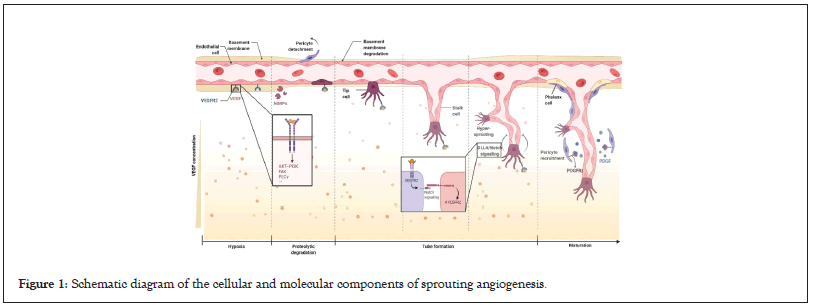
Figure 1: Schematic diagram of the cellular and molecular components of sprouting angiogenesis.
Stimuli such as hypoxia lead to the production of pro-angiogenic factors. The major pro-angiogenic factor VEGF binds to the VEGFR receptor of endothelial cells and promotes endothelial cell differentiation, migration, and extracellular matrix degradation through multiple transduction pathways. The tip of the sprouting capillary points to the direction of secreting factors such as VEGF. During migration, there are mainly two morphologies such as apical cells and stem cells. The interaction of tip cells and stem cells ensures the orderly growth of blood vessels. The basic completion of angiogenesis is Platelet Derived Growth Factor (PDGF) mediated pericyte maturation, extracellular matrix formation, and endothelial cell differentiation (phalanx cell).
The most proangiogenic molecules include growth factors (VEGF, ANG, FGF, etc.), chemokines (TNFα, MCP-1, etc.), and other active molecules (S1P, NO, etc.), among which VEGF is one of the most accepted in promoting angiogenesis. The main downstream signal involved VEGF pathway including other growth factors is PI3K/Akt pathway [17,18]. Thus, PI3K acts as a common downstream signaling transducer in angiogenesis.
VEGFR2 acts as a critical transducer in endothelial cell proliferation biological signals [19]. Among different ligands, Vascular Endothelial Growth Factor-A (VEGFA) is the main factor of angiogenesis, which mainly binds to VEGFR2 with higher tyrosine kinase activity. Most cells can produce VEGFA, including interstitial cells, neutrophils, monocytes, and endothelial cells. VEGFA causes transient vasodilation through the release of Nitric Oxide (NO), which increases vascular permeability, and promotes the proliferation, directional migration, and differentiation of vascular endothelial cells including tip cells, stalk cells, and phalanx cells. Studies have also indicated that VEGFR1 mediates angiogenesis through the inflammation pathway (Figure 2) [20].
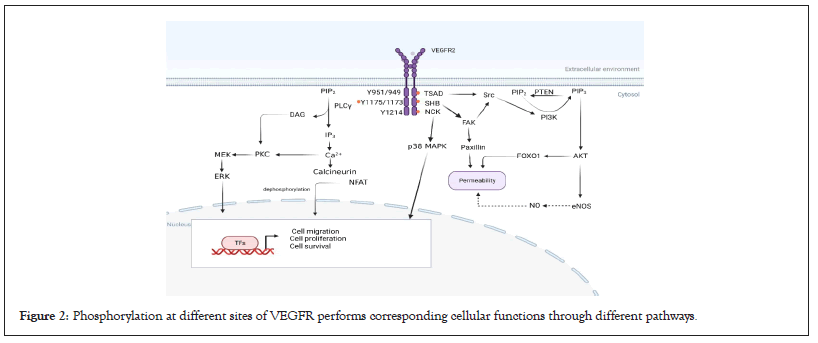
Figure 2: Phosphorylation at different sites of VEGFR performs corresponding cellular functions through different pathways.
Angiopoietin (Ang)/Tie-2 (Tek) pathway is also known as a regulator in vascular development and inflammation. It plays an important role in stabilizing the vascular and promoting angiogenesis. Under physiological conditions, Angiopoietin-1 (Ang-1) binds to and Tie2 receptor, leading to downstream signaling that promotes endothelial cell survival and vascular stability. Angiopoietin-2 (Ang-2) is increased under pathological conditions and acts as a context-dependent agonist/antagonist of the Ang-1/Tie2 axis, resulting in vascular disruption. What’s more, Ang-2 and VEGF-A synergistically drive vascular leakage, neovascularization, and inflammation, key components of retinal vascular diseases [21]. In the animal model of Staphylococcus aureus-induced peritonitis, Ang2-deficient mice cannot produce an inflammatory response. This may be related to increasing intercellular adhesion molecules and the sensitivity of VEGF and TNF-α by increased Ang2. Combined treatment with anti- VEGFA/Ang2 significantly reduced leukocyte infiltration in Choroidal Neovascularization (CNV) mice [22].
Other Receptor for Tyrosine Kinase (RTKs), such as Fibroblast Growth Factor Receptor (FGFR) and Platelet Derived Growth Factor Receptor (PDGFR), also show the same effect as VEGFR. They phosphorylate by their ligand upregulated when Hypoxia- Inducible Factor (HIF) increase. To be more specific, angiogenesis is triggered by the expression of HIF and VEGF, FGF, and MMP etc., [23-26]. And these growth factors act in different roles, for example, VEGF initiates early stages of angiogenesis to promote the formation of primitive tubular structures, while PDGF is believed to stabilize vessels by recruitment of pericytes. Studies have shown that overexpression of VEGFA can interfere with the expression of PDGFRβ, thereby affecting the recruitment of pericytes [27]. Other important receptors in angiogenesis include Delta-like Ligand 4 (DLL4), Notch4, and Ephrin B2, which can interact with the VEGF pathway and regulate angiogenesis field [28,29].
Ocular angiogenesis and its feature: The angiogenesis in the eye also has its own features because of its anatomy. Ocular angiogenesis occurs mainly in the retina, choroid, and cornea, any of which can cause blindness. The retinal Neurovascular Unit (NVU) is the basic unit formed through the interaction of neurons, vascular cells, and glial cells (astrocytes and Muller), maintaining retinal homeostasis. Under conditions such as hypoxia and inflammation, disturbance in NVU communication leads to the breakdown of the Blood Retinal Barrier (BRB) and aggravates the angiogenesis [30]. It is noteworthy that in NVU, pericytes are highly heterogeneous in the tissue. Pericytes themselves can have strong plasticity and regenerative functions and can transdifferentiate into glial cells, etc., [31]. The higher density of pericytes in the retina has been shown to have a restrictive effect on the abnormal value-added of endothelial cells, Thus, unbalanced signals, aberrant metabolisms, and pathological conditions, such as oxidative stress and inflammation, which disrupt communication between pericytes and endothelial cells will affect angiogenesis in retina [32].
Hypoxia-induced expression of VEGF by Retinal Pigment Epithelium (RPE) cells may be a critical link in Choroidal Neovascularization (CNV) development. Alterations in the local microenvironment may cause choroidal capillary occlusion, atrophy, and fibrosis, resulting in inadequate choroidal blood supply. Consequently, retinal ischemia and hypoxia stimulate RPE cells to divide and proliferate and secrete various cytokines such as Vascular Endothelial Growth Factor (VEGF) and basic Fibroblast Growth Factor (bFGF), leading to compensatory growth of choroidal capillaries toward the outer retina and finally the formation of CNV.
Cornea vascular is usually influenced by corneal epithelial cells and corneal limbal stem cells. Abnormalities of stem cells at the corneal limbus are an important factor in pathological neovascularization [33]. Under pathological conditions, capillaries transcend the corneal limbus and enter the corneal clear zone, breaking the vascular pardon of the cornea and causing corneal angiogenesis. Thus, any abnormality of neuro, glial cells, RPE cells, corneal epithelial cells, and corneal limbal stem cells may affect ocular angiogenesis (Figure 3).
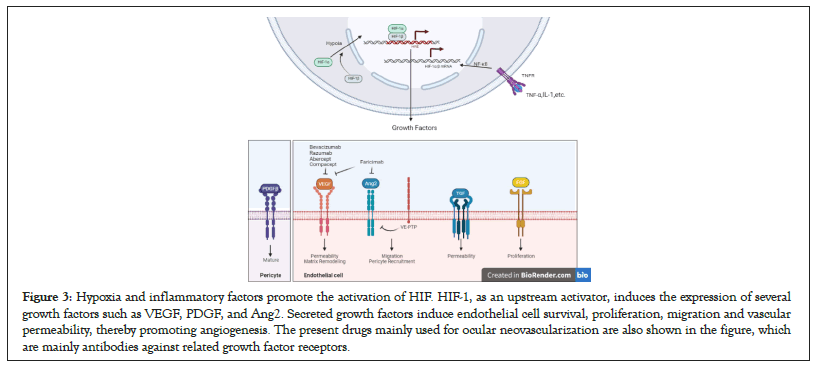
Figure 3: Hypoxia and inflammatory factors promote the activation of HIF. HIF-1, as an upstream activator, induces the expression of several growth factors such as VEGF, PDGF, and Ang2. Secreted growth factors induce endothelial cell survival, proliferation, migration and vascular permeability, thereby promoting angiogenesis. The present drugs mainly used for ocular neovascularization are also shown in the figure, which are mainly antibodies against related growth factor receptors.
Molecular signaling mechanisms in the PI3K pathway
Structure and activation of class I PI3K enzyme: Class I PI3Ks are composed of a catalytic subunit and a regulatory subunit, which respectively regulate the catalytic activity and subcellular localization of the complex. Four class I PI3Ks (PI3Kα, PI3Kβ, PI3Kδ, PI3Kγ) whose catalytic subunits are p110α, p110β, p110γ and p110δ respectively, encoded by PIK3CA, PIK3CB, PIK3CG and PIK3CD genes. The four catalytic subunits are combined with different regulatory subunits. According to different regulatory subunits bound, class I PI3Ks are further divided into class IA (PI3Kα, PI3Kβ, PI3Kδ) and class IB (PI3Kγ). Class IA binds to five regulatory subunits (p85a, p55a, p50a, PIK3R1 encoding; p85b, PIK3R2 encoding; p55g, PIK3R3 encoding), while class IB binds to two other regulatory subunits (p101, PIK3R15 encoding; p84, PIK3R6 encoding). Earlier studies suggested that binding of the catalytic subunit to the regulatory subunit is a non-selective, but recently, studies have shown that p110α and p110β bind non- selectively to p85α and p85β, whereas p110δ seem to bind p85α selectively [34,35]. Class I PI3Ks are tissues specificity, p110α and p110β are widely expressed in mammalian cells, but p110γ and p110δ are mainly expressed in hematopoietic cells [36,37].
All PI3Ks contain a conserved structure called the PI3K core, consisting of a membrane-bound C2 domain, a helical domain, and a kinase domain. All class IA p110 subunits consist of a conserved PI3K core and N-terminal p85 subunit-binding domain and RAS-binding domain (interacting with small G proteins of the RAS family), whereas class IB p110 subunits lack N-terminal p85 subunit binding domain. Class IA PI3K regulatory subunits all contain two SH2 domains (N-SH2, C-SH2), and an inter-SH2 domain (iSH2) between them. The iSH2 domain of the regulatory subunit is linked to the C2 domain of p110s, the N-SH2 domain is linked to the C2, helical, and kinase domains of p110s, and only in PI3Kβ and δ, C-SH2 and the kinase domains are connected [38,39]. This linkage between the class IA catalytic subunit and the regulatory subunit produces contact inhibition, leaving the complex in a low activity state in the basal state. Specific domains are absent from the regulatory subunits of class IB PI3Ks. Recently researchers find that the oncogenic truncations of the PIK3R1 encoded iSH2 domain will activate PI3K signal while mutation of the PIK3R1 encoded C-SH2 domain inhibit it [40]. Besides, free regulatory subunits perform unique functions in cells [41]. Studies have shown that the N-SH2 domain of the regulatory subunit regulates activation and inhibition of dimer via a-loop. These findings reveal insight into the regulation of p110s by regulatory subunit and provide a new direction for PI3K inhibitors (Figure 4) [42].
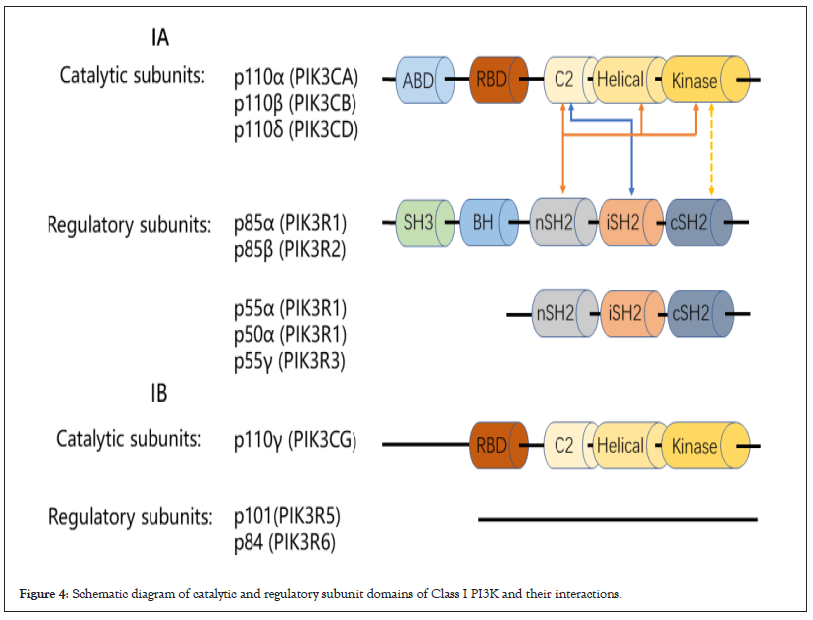
Figure 4: Schematic diagram of catalytic and regulatory subunit domains of Class I PI3K and their interactions.
There are multiple activation mechanisms of class I PI3Ks, including Receptor Tyrosine Kinase pathways (RTKs), small GTPase pathways, and GPCRs pathways. The most typical one is the RTKs pathway, in which the SH2 domain in p85s binds to YXXM phosphorylated tyrosine in Receptor Tyrosine Kinases (RTKs), releasing the contact inhibition between the regulatory subunit and the catalytic subunit, and the activity regulation of class I PI3Ks is ultimately achieved through conformational changes [43,44]. The small GTPase pathway also plays an important role in the activation of class I PI3Ks, and all catalytic subunits of class I PI3Ks have an N-terminal RAS-Binding Domain (RBD) that interacts with RAS proteins from small G proteins, inputting activation signal, but there are exceptions, PI3Kβ is the only isoform of class I PI3Ks that is not regulated by RAS, nonetheless, the RBD of p110β still plays an important role in the activation of PI3Kβ, which can interact with the RHO family GTPases RAC and CDC42 to activate PI3Kβ, and RAC is activated downstream of GPCRs [45]. GPCRs mainly transmit signals through trimeric G proteins, and earlier experiments showed that the Gβγ subunit can directly activate p110β and p110γ, but not p110α and p110δ [46-50].In addition, GPCRs can also indirectly activate p110β and p110γ. The Gβγ subunit can mediate RAC activation through Dock180/elmo1, which subsequently interacts with p110β. Further specific to each PI3K isoform, the activation of PI3Kα mainly depends on the synergy of receptor tyrosine kinase signaling and RAS protein signaling, as does PI3Kδ [51]. Activation of PI3Kγ depends on RAS protein signaling as well as cooperative signaling from GPCRs imported through regulatory subunits [52]. The activation of PI3Kβ is more complex. Compared with PI3Kα, PI3Kβ seems to be insensitive to RTK signaling [53]. Its activation mainly depends on the interaction of RHO family GTPases (RAC and CDC42) with RBD, the direct effect of the Gβγ subunit on p110β, and Gβγ subunit mediated RAC activation through Dock180/ elmo1. However, recent studies in neutrophils have shown that stimulation of GPCRs alone mainly leads to the activation of PI3Kγ, while combined stimulation of GPCRs and RTKs binds Gβγ to p110β, driving the PIP3 response [54].
PI3K effectors: It includes AKT, TEC family and GEFs.
AKT: The physiological function of PI3K is mainly realized by relying on a 57-kDa serine/threonine kinase AKT, also known as Protein Kinase B (PKB). When Class I PI3Ks are activated, it phosphorylates Phosphatidyl Inositol 2 Phosphate (PI2P) to Phosphatidylinositol 3,4,5-triphosphate (PIP3), a lipid second messenger in the cell. AKT is then transported from the cytoplasm to the cell membrane’s surface, where PIP3 specifically binds to the Pleckstrin Homology (PH) domain of the AKT, increasing AKT aggregation to the cell membrane [55]. Three AKT genes (AKT1, AKT2, and AKT3) were discovered in the mammalian genome, each of which performed a particular role in cell physiology and cancer pathophysiology [56,57]. Phosphorylation of AKT can occur at Ser124, Thr308, Thr450, and Ser473 [58]. The traditional phosphorylation of this protein is mediated by PDK1, a 63-kDa protein with a PH domain and a kinase domain. On the one hand, PDK1 can bind tightly to PIP3 and then transfer to the cell membrane surface; on the other hand, when PDK1 is transferred to the cell membrane, PIP3 can bind to the PH domain of AKT, causing conformation of AKT to alter and PDK1 to phosphorylate Thr308. Furthermore, mTOR Complex-2 (mTORC2) phosphorylates AKT Ser 473. Overall, PDK1 can phosphorylate AKT directly and mediate the second messenger PIP3, which is important in a variety of signaling pathways [59].
The main function of AKT is to suppress apoptosis and act as a key signaling molecule for cell development and differentiation. AKT reduces cell apoptosis by phosphorylating BAD, a member of the Bcl-2 family, which impairs its capacity to attach to Bcl-XL and inhibits cell death [60]. AKT can also prevent the activation of the apoptotic cascade by inhibiting the action of the proteolytic enzyme caspase-9 [61]. AKT can also activate IKB Kinase (IKK), which causes IKB to be degraded, allowing NF-kB to be released from the cytoplasm and translocated to the nucleus, where it activates its target oncogenes to drive cell proliferation [62,63]. AKT can also phosphorylate several proteins that are involved in glucose metabolism [64]. FOXO1 is a forkhead transcription factor that regulates the expression of genes involved in glucose metabolism, apoptosis, cell cycle arrest, and other cellular processes. FOXO1 can be phosphorylated by AKT, which prevents its nuclear translocation and transcriptional activity [65]. AKT1 is found mostly in vascular endothelial cells and causes aberrant blood vessels, eventually leading to tumor vascular abnormalities [66]. What’s more, VEGF controls its receptor expression via the Akt signaling system by activating HIF-1, which controls numerous genes, including VEGF and several glycolytic enzymes required for angiogenesis [67]. Other angiogenic agents, such as Nitric Oxide (NO) and Angiopoietin (ANG), are similarly regulated by the PI3K/Akt pathway. To be more specific, AKT promotes tumor angiogenesis by activating endothelial NO, inducing NO- dependent vasodilation and endothelial cell migration, whereas, only in the presence of VEGF, angiopoietins (ANG1 and ANG2) can be promoted [68].
TEC family: The TEC family of tyrosine kinases is the important downstream effectors of PI3K in B cells. Among them, BTK, ITK, and TEC all have Pleckstrin Homology (PH) domains, which can be recognized and combined with PtdInsP3 with high affinity. Bone marrow kinase in chromosome X (BMX), also known as ETK, is a member of the TEC family and is expressed mainly in arterial endothelial. It is documented that BMX regulates ischemia-mediate angiogenesis while not affecting the developmental vascular growth [69].In BMX-deficient mice, BMX was required for full phosphorylation of the Mitogen Activated Protein Kinases (MAPK) p38 and JNK which might mediate cell proliferation. In a mouse knockout research, it was discovered that mice lacking Pik3r1 or Pik3cd have symptoms identical to mice lacking the BTK gene, indicating that PI3K- dependent regulation of BTK translocation to the plasma membrane is essential for BTK function [70]. Studies have shown that BTK can maintain the number of Cancer Stem Cells (CSCs) in Glioblastoma (GBM), and CSCs can produce higher levels of VEGF under both normoxia and hypoxia than non-CSCs, promote vascular proliferation and tumor growth, while the use of BTK inhibitor acalabrutinib can reduce GBM cell viability, negatively regulate CSCs, control the expression of VEGF, and finally inhibit angiogenesis [71-73].
GEFs: The GEF family consists of approximately 80 members. The GEF proteins usually contain a Dbl homology (DH) domain and a PH domain that is important for plasma membrane localization, where the activation of Rho GTPases takes place. Within blood vessels, Rho GTPases and Guanine nucleotide Exchange Factors (GEFs) regulate endothelial cell adhesion in tightly barrier function which is most related to vascular development, angiogenesis, and migration of inflammatory cells. The cellular signaling of cytoskeletal remodeling is triggered or enhanced by small GTPases which are controlled by GTPase Activating Proteins (GAPs) and GEFs. Among GEFs, P-Rex1 has a unique PH domain that can operate as a significant downstream effector of the PI3K factor.
Expression of the P-Rex1 in endothelial cells mediates Stromal cell-derived Factor 1 (SDF-1) induced Rac1 signaling that controls cell adhesion and migration [74]. In neutrophils, P-Rex1 is co- activated by the βγ subunit of a heterotrimeric G protein with PIP3, which subsequently stimulates the GTPase cascade including RhoG and Rac, drives Rac-mediated actin recombination, and leads to the migration of neutrophils [75]. Therefore, the PI3K/ Rac signaling axis can regulate cell morphology and motility [76]. Hypoxic tumors can secrete Stromal cell-derived Factor-1 (SDF- 1) and VEGF to promote angiogenesis. Studies have shown that P-Rex1 knockout cells have reduced migration and angiogenesis upon SDF-1 stimulation, but P-Rex knockout does not affect the response to VEGF, thus P-Rex1 is SDF-1-induced angiogenic response key facilitators (Figure 5).
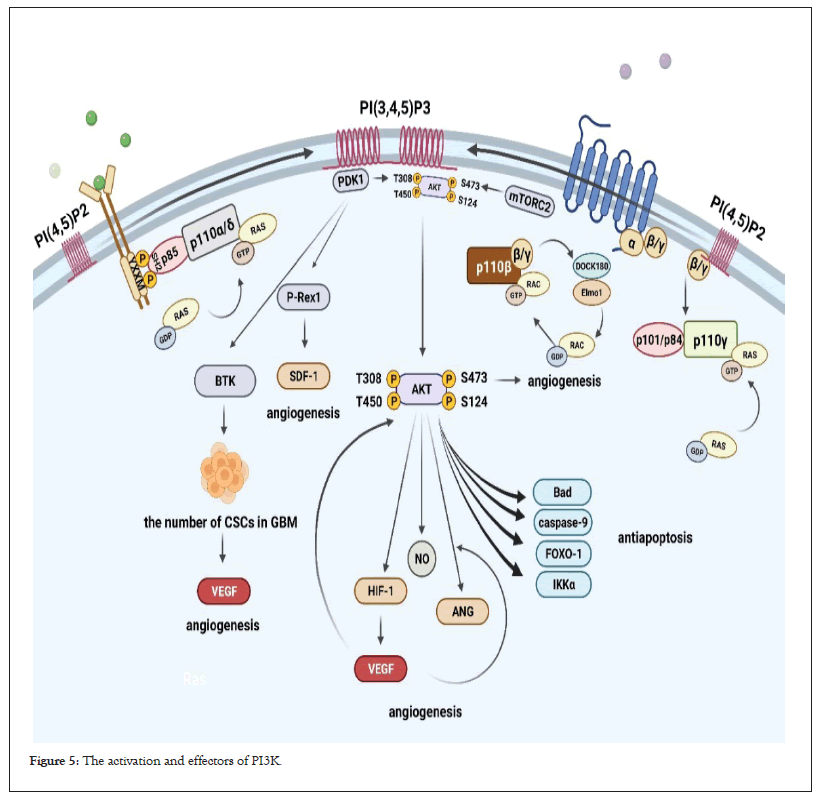
Figure 5: The activation and effectors of PI3K.
The activation of PI3Kα and PI3Kδ mainly depends on the synergy of receptor tyrosine kinase signaling and RAS protein signaling. Activation of PI3Kγ depends on RAS protein signaling as well as cooperative signaling from GPCRs imported through regulatory subunits. Compared with PI3Kα, PI3Kβ seems to be insensitive to RTK signaling. Its activation mainly depends on the interaction of RHO family GTPases (RAC and CDC42) with RBD, the direct effect of Gβγ subunit on p110β, and Gβγ subunit mediated RAC activation through Dock180/elmo1; PIP3 binds to PI3K downstream effector AKT, TEC family tyrosine kinase BTK and P-Rex1 of Guanine nucleotide Exchange Factors (GEFs); after PDK1 binds to PIP3, it phosphorylates the Thr308 site of AKT; mTORC2 phosphorylates the Ser473 site of AKT; AKT phosphorylates BAD, a member of the Bcl-2 family, proteolytic enzyme caspase-9, Forkhead transcription factor FOXO1 and IkB kinase (IKKα) to play an anti-apoptotic role; AKT activates NO and ANG, then interacts with VEGF to promote angiogenesis and BTK maintains the number of Cancer Stem Cells (CSCs) in Glioblastoma (GBM) then maintains high levels of VEGF in tumors to promote angiogenesis; P-Rex1 is a key promoter of SDF-1-induced angiogenic responses.
Modulation of PI3K signaling: PI3K signaling pathway is antagonized by some lipid phosphatases, such as Phosphatase and Tensin homolog (PTEN) and SHIP. PTEN is known as a tumor suppressor gene, and mutations of PTEN are observed in a variety of genetic diseases and tumors [77]. It is thought to function primarily as a PIP3 3-phosphatase, and it can dephosphorylate Phosphatidylinositol 3,4,5-trisphosphate (PIP3) to Phosphatidylinositol 4,5-bisphosphate (PIP2), blocking phosphorylation of PI3K effectors, hence negatively regulates the PI3K signaling cascade. Artificially introduced mutations in PTEN lead to enhanced cell proliferation and migration thus promoting neovascularization and tumor metastasis. And this effect is partially dependent on the PI3K subunits p85α and p110γ, which reveal the important role of PTEN in the PI3K pathway regulation [78]. The 5-phosphatases acting on PIP3 are mainly SHIP1 and SHIP2, and SHIP2 is involved in insulin signaling and glucose homeostasis [79]. The relative flux and physiological significance of PIP3 through 3 and 5-dephosphorylation is currently unknown [80]. PTEN appears to be the master regulator of class I PI3K signaling in angiogenesis, and the functional relevance of SHIP in endothelial cells is unclear [81].
In addition, increasing evidence suggests that non-coding RNAs play a significant role in the regulation of the PI3K signaling pathway. MicroRNAs (miRNAs) are short non-coding RNAs with critical function in regulation at the post-translational level of the whole genome, and a recent review summarizes its role in tumorigenesis and progression through its crosstalk with PI3K signaling pathway [82]. Circular RNAs (circRNAs), a newly discovered type of noncoding RNA, act as competitive endogenous RNAs (ceRNAs) of microRNAs (miRNAs) in tumor progression by interacting with the PI3K/AKT pathway [83]. The discovery of these noncoding RNAs provides a new direction for the development of PI3K inhibitors.
Biological function of PI3K in angiogenesis
Vascular permeability: The disability of vascular permeability is involved in many physiological and pathological processes [84]. Angiogenesis is usually accompanied by changes in vascular permeability, and the tightness of junctions between vascular endothelial cells is regulated by a variety of mediators, including VEGF, NO, TNF- α, and cytokines and most of these mediators are regulated by the PI3K signaling pathway [85-87]. VEGF stimulates the phosphorylation of VEGF receptor-2 (VEGFR-2), which increases vascular permeability and endothelial cell fenestrations by activating Rac, while PI3K acts upstream of Rac in the VEGF/VEGFR-2 signaling pathway [88]. The Receptor Activator of Nuclear factor receptor-ĸB Ligand (RANKL) is an important regulator of vascular pathophysiology, and RANKL stimulates eNOS through the TRAF6-PI3K-AKT pathway, thereby promoting vascular permeability and angiogenesis [89]. In addition, a study in glioblastoma found that PI3K/AKT-mediated phosphorylation and delocalization of the cell-cell adhesion junction protein Afadin may play an important role in regulating the permeability and migration of brain endothelial cells [90]. It was also found that mice carrying PTEN-deficient mast cells exhibited increased vascular permeability [91]. Sphingosine-1 Phosphate Receptor-2(S1PR2) increases paracellular permeability via the PI3K signaling pathway [92]. These findings further confirmed the importance of PI3K signaling in the regulation of vascular permeability.
Furthermore, different class I PI3K isoforms play unique roles in vascular permeability. Among the class I PI3K isoforms, p110α selectively regulates endothelial barrier function, and compared with other PI3K isoforms, the PI3Kα-selective inhibitor PI-103 is most effective in reducing endothelial cell permeability [93]. PI3Kα may regulate Pyk2- and RAC1-mediated endothelial function by inducing the formation of VE-cadherin-related protein complexes [94]. And one study assumes that activation of RhoA mediates vascular permeability in capillary malformation- arteriovenous malformation syndrome, while RhoA can be activated by p110α [95]. PI3Kδ is involved in the VEGF-mediated increase in microvascular permeability, and inhibition of PI3Kδ inhibits the increase in the microvascular permeability [96]. Vascular leakage caused by IL-8 necessitates the CXCR2/Rac1 signaling axis, which PI3K is an essential part of [97]. However, there is a study reporting that Anti-PI3K (α/β) therapy inhibits VEGF-induced responses in the body and the viability of primary human endothelial cells in culture, and inhibition of PI3K (γ/δ) inhibits VEGF-mediated vascular permeability in vivo without affecting the survival of primary endothelial cells, which suggests that angiogenesis and vascular permeability may be independently regulated downstream of VEGF [98].
PI3K in angiogenic sprouting: Sprouting is an early step in this complex program of vascular network formation. Tip cells possess numerous long and motile filaments that extend along concentration gradients of proangiogenic factors and pair to achieve orientation. In the postnatal mouse retina, glial cells secrete VEGF to form concentration gradients that promote tip cell development with specific transcriptional signatures and express various proteins that mark their identity [99,100]. What’s more, the positive and negative feedback of tip cells on nearby cells is also a major pathway for the directional growth and maintenance of the orderly generation of blood vessels. A subset of endothelial cells differentiates into a tip cell phenotype, whereas other ECs are stalk cells or square cells of the posterior processes to maintain the integrity and perfusion of the vascular bed. This decision is strongly controlled by the Notch pathway, Delta-Like Ligand 4 (DLL4), which, by transactivating the Notch pathway, inhibits the development of tip cell properties in adjacent stalk cells, forming their expression and function [101].
PI3K regulates angiogenesis with isoform selectivity. Class I PI3Ks, especially p110α, are indispensable in early embryonic angiogenesis by regulating endothelial cell proliferation, migration, and morphogenesis. Pervasive knockout of p110α results in delayed embryonic development and defective angiogenesis in the second trimester [102,103]. Disrupting the interaction between RAS and p110α in a mouse model of tumor cell metastasis can inhibit tumor growth and tumor-induced angiogenesis [104]. At the same time, many researchers have found that there are PIK3CA gene mutations in the vascular malformations and lymphatic malformations, and these gene mutations usually occur in the vascular endothelial cells, which further confirm the important role of PI3Kα in angiogenesis [105-107].
p110α activity is particularly high in endothelial cells and mainly induced by tyrosine kinase ligands (such as VEGF-A). Several studies have demonstrated that PI3Kα signaling mainly regulates cell migration during angiogenesis in mice and zebrafish, and p110α is a positive regulator of the small GTPase RhoA, suggesting that p110α controls endothelial cell migration by regulating RhoA activity [108]. Subsequent studies further found that the activity of RhoA is regulated by ARAP3 [109]. ARAP3 is a specific RhoA GAP, and it is also a PI3K effector, and its GAP activity is dependent on PIP3, and its N-terminal PH domain mediates its interaction with PIP3 [110,111]. Knockout of the ARAP3 gene in mice resulted in severe vascular defects that closely resembled the phenotype observed when p110α was inactivated. Further researchers introduced point mutations in the ARAP3 N-terminal PH domain (R302, 303A) that interfered with the regulatory input of PI3K to ARAP3, also resulting in angiogenesis abnormalities in mice. These results suggest that ARAP3 performs a critical function in PI3Kα-regulated angiogenesis. In addition, studies have also shown that the above process is regulated by the Class II PI3K PI3K-C2a, PI3K-C2a knockdown will impair VEGFR internalization and endosomal RhoA activation [112]. Furthermore, studies have also shown that endothelial cell rearrangements during vessel growth require PI3Kα-mediated inhibition of actomyosin contractility through AKT-independent PI3K/NUAK1/MYPT1/MLCP pathway [113]. Previous studies believed that p110β was functionally overlapping with p110α and p110γ, but recent studies have shown that it has a unique role in signal transduction. Studies have shown that the inactivation of p110β can inhibit PI3Kα/Akt signaling, thereby inhibiting the migration of vascular endothelial cells, which is also associated with decreased expression of the angiogenic tip cell markers apelin and DLL4 [114,115]. In particular, studies have shown that S1P-induced endothelial cell migration mainly depends on p110β-stimulated Akt [116].
PI3K in pericyte maturation: Pericytes are required for the stabilization of the barrier of brain retina blood and have been shown in numerous studies to be critical in the permeability of vascular which is an initial step in angiogenesis. The integrity of pericytes around endothelial cells is regulated by a series of signaling molecules, such as VEGF, PDGFB, Notch, Ang, and TGF-β, etc., [117]. Among these molecules, PDGFB/PI3K pathway is required for the proliferation and migration of pericytes for vascular formation. Then S1P from pericytes binds to receptors in ECs to facilitate adhesion between endothelial cells and the ECM [118,119]. Recent studies have shown that there is high PI3K signal transduction in immature pericytes and the persistent high signal PI3K pathway hinders pericyte maturation, which is an important cause of abnormal angiogenesis. This is also related to the abnormal regulation of the PTEN [120-122].
In early studies, PI3Kα has been implicated in regulating pericytes in angiogenic processes. However, among the patients with PI3Kα mutation, partly the treatment targeting PI3Kα induced resistance, which can be reversed by the inhibition of PI3Kβ [123]. As mentioned above, as a member of the class I PI3K family, PI3Kβ is ubiquitous in body cells. PI3Kβ is the only known isotype in the family that is simultaneously activated by RTKs via the p85 linker subunit and GPCRs via the Gβγ subunit [124]. Previous studies believed that it overlaps with p110α and p110γ in function. But the reversal of resistance by PI3Kβ inhibition suggested that it has a unique role in the signal transduction. Similarly, some research discussed that the combination of PI3Kα and PI3Kβ inhibition is also more effective at restraining the angiogenesis [125,126]. Yet the role of PI3Kβ is not clear.
In the past few years, some researchers have proposed a point that the inactivation of p110β has a unique role in pericyte maturation, which may be related to Gβγ, Rac1, and Rab5. Crossbreed Pdgfrb(BAC)-CreERT2 mice with RiboTagflox/flox mice to assess morphological changes in pericytes of retina. They found that inherited PI3Kβ inactivation triggers early maturation of pericytes. Conversely, the release of PI3K signaling through PTEN deletion delayed the pericyte maturation [127,128]. In particular, studies have shown that S1P-induced endothelial cell migration mainly depends on p110β-stimulated Akt, and p110γ plays a coordinated part in EC migration toward the chemotactic gradient [129]. This evidence suggests that different types of upstream signals are required to stimulate cell proliferation in pericytes through the activation of PI3Kβ, thus providing new avenues for the treatment of pathological angiogenesis. Note also that only targeting PI3Kβ maybe not be enough because the efficacy is transient according to the study.
PI3K meditate angiogenesis through inflammation: There has been a lot of work regarding the regulating VEGF level by the PI3K pathway, which mechanistically induced the HIF-1 [130]. PI3Kα has been reported to be the crucial isoform regulating VEGF levels in angiogenesis. Now there is more evidence suggesting that angiogenesis is a complex process, requiring the involvement of inflammatory cells [131,132]. Even though all class I PI3Ks are expressed by inflammatory cells, the two isoforms of PI3Kγ and PI3K are preferentially expressed in the immune system and exert selective anti-inflammatory actions in different immune cell groups [133]. In this section, the role of the two isoforms PI3Kγ and PI3Kδ in neutrophils and macrophages are discussed in the following sections.
Macrophage recruitment and polarization is one of the first steps in the development of inflammation. This process leads to not only the pro-inflammatory cytokine and VEGF production but only the hypoxia reaction in a positive feedback manner. The early research investigated the p110b or p110δ in macrophages regulates the colony-stimulating factor-1 [134]. Especially, a recent study has shown that M2 macrophages release Arg1, VEGF, and activate STAT3, playing a key role in regulating retinal pathological angiogenesis. Idelalisib, a selective inhibitor of PI3Kδ, has recently been extensively studied in lymphoma. In addition, some studies have also shown that Idelalisib injected into the vitreous can inhibit the monocyte polarization [135,136]. Similarly, Eganelisib targeting the PI3Kδ promotes the reprogramming of M2 into M1 to reduce tumor angiogenesis. As for the role of PI3Kγ, numerous studies have confirmed a correlation between the inactivation of macrophage PI3Kγ and tumor angiogenesis [137]. The mechanism, it is capable of restoring CD8+T cell function in vitro by inducing an immunostimulatory transcriptional program. In some chronic inflammatory diseases, the study has substantiated the loss of PI3Kγ rarely resulted in the infiltration of macrophages. The PI3Kγ related infiltration is activated by the extracellular matrix degradation product in a positive feedback way [138].
Neutrophils like macrophages are important in the inflammatory process. In a lung inflammatory paradigm, Wild-type (WT) mice treated with IC87114, a selective inhibitor of PI3Kδ, had fewer neutrophil invading cells and an impaired capacity to migrate in a directed manner [139]. Migration is reduced in the absence of PI3Kγ expression in response to certain GPCR activation [140- 143].
Compared with γ, δ is more common in lymphocytes. Previous studies have shown that PI3Kδ has a critical role in untransformed B cells and that B cell antigen receptor signaling is almost entirely dependent on PI3Kδ compared to other isoforms of PI3K. Activation of BCR phosphorylates intracellular ITAM and promotes SYK activation. Activation of SYK binds to the p85 subunit of PI3Kδ and regulates the expression of proinflammatory factors through the PI3K/AKT/mTOR pathway [144]. While recent studies suggested that although the expression of PI3Kδ is at low levels in other cells including ECs, TNF-α induces p110δ expression in ECs, increasing the expression of VEGF. The resultant upregulation of inflammation plays an important role in diabetic retinopathy, which is a novel perspective in the pathological angiogenesis. Unexpectedly, as for Tregs, the inactivation of PI3K-δ and PI3K-γ could enhance the anti-lymphoma immune response [145]. Among the lymphoma patients administrated with Idelalisib, colitis is more common and has been studied to be associated with effector T cell infiltration [146-148].
In conclusion, PI3Kδ/PI3Kγ inhibitors reduce inflammation- mediated angiogenesis. Despite of their well-defined roles in immune cells, the exact role of PI3Kδ and PI3Kγ in angiogenesis in immune cells and other cells (fibroblasts, endothelial cells, etc.,) remains to be determined. In addition, immune activation can also be observed when using inhibitors, which reminds us to pay more attention to the side effects of drugs.
Role of PI3K in ocular vascular disease
Retinal vascular disease: It is thought that angiogenesis in retinopathy begins with the activation of retinal endothelial cells by a stimulus such as hypoxia and that this activation is mediated by the action of angiogenic cytokines usually. VEGFR has been shown to be widely expressed in retinal endothelial cells, pericytes, glial cells, and pigment epithelial cells, and the activation of VEGFR can alter vascular permeability and induce the release of proinflammatory and proangiogenic mediators from macrophage microglial. Thus VEGF has become the main therapeutic target for PDR today, however, resistance to anti-VEGF drugs or disease relapse has been observed in a considerable number of PDR patients, and there is an urgent need to find new therapeutic targets [149]. PI3K is located downstream of the VEGF signaling pathway, and PI3K may become a new therapeutic target for PDR. Numerous studies have shown that the PI3K/AKT pathway is involved in the angiogenesis, and these studies also show that PI3K/Akt signaling can activate the MAPK and VEGF pathways, which in turn promote cell proliferation and angiogenesis [150-152]. In this process, both the PI3K/Akt and the MAPK signaling pathways can promote the expression of HIF-1 [153]. HIF-1 responds to hypoxia signals and upregulates the Vascular Endothelium Growth Factor (VEGF) signaling pathway, which in turn activates the Mitogen Activated Protein Kinase (MAPK) signaling pathway, resulting in increased cell proliferation and angiogenesis. Recent studies have shown that this pathway also plays a role in retinal neovascularization. The Lectin Galactoside- binding soluble 3 Binding Protein (LGALS3BP) in microglia promotes retinal angiogenesis through PI3K/AKT pathway during hypoxia. Pigment Epithelium Derived Factor (PEDF), a 50-kDa protein secreted by the retinal pigment epithelium, can inhibit VEGF-mediated PI3K/Akt phosphorylation, thereby inhibiting the migration and tubule formation of RECs, and inhibit retinal neovascularization [154,155]. Apelin/APJ signaling pathway is a key factor for hypoxia-induced pathologic angiogenesis and it is regulated by PI3K [156]. Ag-NPs is a potent anti-angiogenic molecule that inhibits retinal neovascularization by inhibiting PI3K/Akt-depending VEGF-induced migration and tubule formation of bovine retinal endothelial cells in a manner similar to PEDF [157,158]. These findings reveal the role of PI3K signaling in retinal pathological neovascularization. However, studies involving specific PI3K isoforms are scarce and further research is needed.
Choroidal vascular disease: Choroid between the retina and the sclera is mostly made up of blood vessels. Its thickness fluctuates a lot depending on how full the blood vessels are. Its primary role is to nourish the retina’s outer layer while blocking light from entering the eye through the sclera. Choroidal Neovascularization (CNV) is the ultimate symptom of almost 40 ocular illnesses, including Age-related Macular Degeneration (AMD), which can result in severe vision loss [159,160]. Age, smoking, genetics, and cardiovascular disease are all known risk factors for CNV [161- 164]. Anti-VEGF inhibition is the current standard of therapy for CNV, and it can considerably reduce CNV-induced blindness.
PI3K signaling pathway also plays an important role in choroidal neovascularization. Cyr61 is a critical cell-matrix regulator in the regulation of cell adhesion, migration, proliferation, angiogenesis, inflammation, and tissue remodeling [165-171]. Advanced Glycation End products (AGEs) have been shown to increase the expression of Cyr61 in Retinal Pigment Epithelium (RPE) cells in diabetic patients, which activates the integrin- PI3K/AKT signaling pathway and promotes the expression of VEGF in RF/6a cells, ultimately promoting the progression of CNV [172]. PKR is a serine-threonine protein kinase whose phosphorylation is regulated by interferon-induced double- stranded RNA (dsRNA) and has antiviral, anticancer, and immunological regulatory functions [173]. PKR, as an upstream regulator of VEGF, has been found to stimulate the downstream PI3K/AKT signaling pathway, upregulate VEGF production, and increase CNV progression [174].
The most prevalent primary intraocular malignancy in adults is Choroidal Melanoma (CM), which has a high incidence of malignancy and distant metastases. Angiogenesis is thought to be a crucial element in tumorigenesis as tumor microcirculation is important in CM metastasis and tumor blood supply. In 1999, however, a notion known as angiogenic Vascular Mimicry (VM) was proposed by, which claimed that invasive cells may construct vascular channels that increase tumor blood supply without the need for tumor angiogenesis. Instead of endothelial-like tubular structures connecting to host blood arteries associated with angiogenesis, VM describes vascular channels generated by highly aggressive and genetically dysregulated tumor cells [175]. The expression of VEGF, which binds to VEGF receptor 1 (VEGFR1) and VEGF receptor 2 (VEGFR2), is linked to the creation of VM [176]. The study found that VEGF activates the PI3K/AKT signaling pathway, which leads to the production of VM in CM [177]. Besides, VEGFR2 promotes the PI3K/AKT signaling pathway, and PI3K in turn activates downstream enzymes such as MT1 MMP and MMP-2.
Corneal vascular disease
Corneal neovascularization is an increasingly serious public health problem, and need corneal transplantation ultimately. The main feature of a normal cornea is no blood vessels. Pathological conditions such as infection, trauma, and transplant rejection will destroy the balance between corneal angiogenesis and antiangiogenic factors, and induce the process of angiogenesis [178]. Common corneal diseases leading to corneal neovascularization include herpetic ophthalmopathy, infectious keratitis, corneal ulcer, chemical burn, graft rejection, and anoxia damage caused by wearing contact lenses [179]. Various promoting factors VEGF, PDGF, and ANG1/2 are involved in corneal neovascularization. As mentioned earlier, PI3K is a signal transduction pathway both upstream and downstream of these promoters.
In the mouse model of inducing corneal neovascularization, using PI3K inhibitors wortmannin and LY294002, compared with untreated mice, the number of neovascularization was significantly reduced and compared with the corresponding control, expression of VEGF, Ang1, Ang2, and PDGF decreased [180]. On the side, PTEN is involved in resisting the PI3K pathway, and PTEN and PI3K inhibitors picolinate, bpv(pic), and LY294002 can significantly improve the cell migration rate in Human Corneal Epithelial (HCE) cell monolayer and rat corneal scratch model [181]. In the model of corneal injury, VEGFR2 and STAT3/PI3K/Akt signaling pathways are down-regulated by using related VEGF receptor antagonists to inhibit the corneal neovascularization. Anti-VEGF drugs are currently the main early treatment of corneal neovascularization. However, many studies have shown that VEGF inhibitors such as bevacizumab have significant effects in the early stage of neovascularization, but not in mature vessels, which indicates that anti-VEGF needs to be used in active neovascularization that depends on angiogenic factors. Studies have shown that the increase of MMP-14 in the MMP family is related to PI3K-mediated inhibition of VEGF and PDGF pathways, and it can also inhibit PI3K/Akt pathways by increasing PTEN expression, thus inhibiting angiogenesis [182].
In the past 20 years, there is an enormous development of PI3K from basic research to clinical application. The chemical reaction of inositol phosphorylation plays a main role in intracellular signal transmission. Much progress has been made in understanding the roles and mechanisms of action of the different PI3K isoforms. However, it is a challenge to verify the function of specific PI3K isoforms in the precise disease, especially ocular angiogenesis, Furthermore, selective inhibitors might provide the benefits of blocking each isoform individually but avoiding complete immune suppression cannot be ignored, this will be a novel strategy to maximize the effect of PI3K inhibitors as antiangiogenics. Here we describe more detail in how the PI3K isoform participates in ocular angiogenesis.
The graphical abstracts were created with BioRender software. This work was supported by the National Natural Science Foundation of China (81900893 to W.W), Science and Technology Plan Project of Hunan Province (2019RS2011 to W.W), The Natural Science Foundation of Hunan Province (No.2021JJ41030 to W.W ), Open Project of State Key Laboratory of Ophthalmology (303060202400374 to W.W).
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Lai D, Liu B, Xiong L, Yin Y, Xia X, Wu W, et al. (2023) The Role of PI3K Isoforms in Ocular Angiogenesis. J Clin Exp Ophthalmol. 14:955.
Received: 01-Jun-2023, Manuscript No. JCEO-23-24712; Editor assigned: 05-Jun-2023, Pre QC No. JCEO-23-24712 (PQ); Reviewed: 19-Jun-2023, QC No. JCEO-23-24712; Revised: 26-Jun-2023, Manuscript No. JCEO-23-24712 (R); Published: 03-Jul-2023 , DOI: 10.35248/2155-9570.23.14.955
Copyright: © 2023 Lai D, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.