Journal of Probiotics & Health
Open Access
ISSN: 2329-8901
ISSN: 2329-8901
Review Article - (2021)Volume 9, Issue 2
The ambient environment contains small plastic materials that can negatively influence the health of living organisms. As the plastic production rate annually increases, many plastic molecules can be degraded by different environmental factors. Thus, the plastic possesses the ability to penetrate the cells of living organisms and harms living organisms through different animal feedstuff and fish. The negative effect of nano-plastic can be noticed in many diseases, nutritional disorders, and the growth rate. Moreover, nano-plastic is a major source of toxins. We revised the possible action of dietary fibers to decrease the toxic effects of non-plastics, based on a large portion of undigested fibers in monogastric to excrete such nano-molecules. In addition, we explained the dietary fibremicrobiome synergistic axis to reduce the risk of plastic in the digestive tract. Finally, we highlighted related research gaps related to the herein review.
Dietary fiber; Feedstuff; Microbiota; Nano-plastic; Toxins
MPS (Micro-plastic Particles); NPS (Nano-plastic Particles); DF (Dietary Fiber); VF (Volatile Fatty acids); IDF (Insoluble Fiber); SDF (Soluble Fiber); SCAFAs (Short-Chain Fatty Acids); FSP (Food Stamp Program); MCFAs (Medium-Chain Fatty Acids); μg (Micro Gram); Mm (Millimeter); PE (Polyethylene); PP (Polypropylene); PVC (Poly Vinyl Chloride); PET (Poly Ethylene Terephthalate); PS (Polystyrene); FFAR 2,3 (Free Fatty Acid Receptors 2,3); GPCR 3,4 (G Protein-Coupled Receptors 3,4); LDLC (Low-Density Lipoprotein Cholesterol); IBD (Inflammatory Bowel Disease); PAHs (Polycyclic Aromatic Hydrocarbons)
The global plastic production has been increased from 0.5 to 322 million tons (Plastics Europe, 2017). As plastic production increases, the amounts of plastic wastes that discharged into the marine environment increase [1-3]. The amount of plastic in the oceans is expected to reach 250 million metric tons by 2025 [4,5]. Nano-plastics are particles within a size ranging from 1 to 1000 nm resulting from the degradation of industrial plastic objects and can exhibit a colloidal behavior where plastic materials break down into nano particles and resemble a colloidal solution, and thus are difficult to distinguish microscopically [6]. The density of nano-plastic is 1.4 g/cm3. There are many concerns about Nano and feeds. The amount of Nano and micro-plastic in the aquatic environment rises due to the industrial production of plastic and the degradation of plastic into smaller particles. Concerns have been raised about their incorporation into food webs [7]. The micro-plastic particles are directly consumed by marine creatures such as fish which may be used in the rations of poultry and pigs, or in human food. For this reason, decreasing the bad effects of nano plastic on animals and feeds has a great importance to reduce the harmful effects of nano-plastics on living organisms. These particles possess a potential toxicity in both nano and microforms due to their inherent ability to induce intestinal blockage or tissue abrasion. In this review, we revised the effects of nanoplastics and micro plastics on living organisms. We highlighted the possible mechanisms of micro and nano-particles to enter the food and feed chains, and the opportunities to decrease their effects using dietary fibers through the gastrointestinal tract.
The effects of nano-plastic on animal health
These particles possess a potential toxicity in both Nano and forms due to their inherent ability to induce intestinal blockage or tissue abrasion. On the other hand, micro-plastic particles (MPs) are most hazardous due to their capability of crossing biological barriers in the organisms [8]. Furthermore, there are many negative effects of micro-plastic particles (MPs) on the cells of animals including, intestinal blockage, physical damage such as the liver and the circulatory system and thus transfer their effects to the rest of the body, histopathological alterations in the intestines, damaging the liver, lipid metabolism, and the immune system [9]. All these symptoms were are found in the whole body of organisms.
The negative effects of Micro-plastic Particles (MPs) on the cells of animals
Many negative effects of MPS on animal cells were noticed as including, immune system suppression, decreased host resistance to infectious agents and tumors, inactivation, increased risk of developing allergic and auto-immune diseases, tissue or organ damage and dysfunction of organs. It was expected that diseases related to Gastro-Intestine Tract (GIT) could be potentially worsened since most of the particles may be deposited in the GI tract and may interact with bioprocesses. Furthermore, some studies on plastic particles showed that they can enter the lymph nodes. Thus will effect on homes, and can badly affect the immune system, Feeding activity [10-13]. Reproduction, oxygen consumption rates [13,14]. Metabolic abnormalities in organisms [15,16]. Besides, disturbances in micro biota morphology, these factors may directly or indirectly affect the gut and the immune system as well as engaged by endocrine disruptors in the organisms growth inhibition behavioral disorders, reproductive [17-19]. Nano-plastics have a small volume and a relatively large surface area. Through complete enzymatic hydrolysis, PET can be degraded directly to Terephthalic Acid (TPA) and Ethylene Glycol (EG) as indicated by the solid arrow on the far right. As well the dashed arrows represent multiple steps. Therefore, some evidence about the penetrating of nano plastic particles in animals and feeds are shown in Figures 1 and 2 [20].
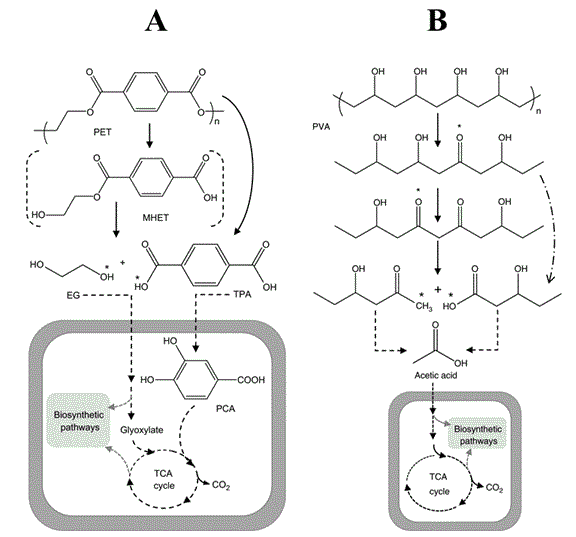
Figure 1: (A) Proposed pathway for the degradation of Polyethylene Terephthalate (PET). (B), proposed biodegradation pathway of Polyethylene (PE). Adapted from [21].
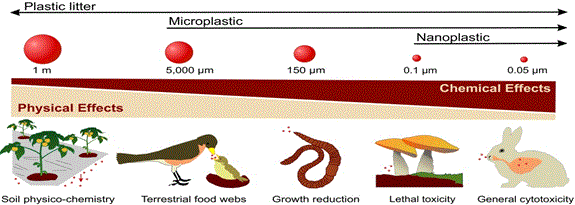
Figure 2: Micro-plastics as the trigger of combined physical or chemical-like effects, Soil biogeochemistry related to agricultural mulching [22], ingestion by terrestrial and continental birds[23,24], Reduction in the growth of earthworms [25], Lethal toxicity to fungi [26] mammal lung inflammation [27], and broad cytotoxicity of plastics [28,29]. Adapted from [30].
Nano plastics penetrate the gills, liver, and the brain in marine animals [31-33]. The microplastics were (124-438 μm) in the liver of the European anchovies [32]. On the other hand, the nanoplastic particles enter the stomach and intestine in the human by other different ways such as honey, beer, salt, and other food materials [34,35]. Micro-plastics with size ≤ 20 μm can penetrate organs such as liver and giblets while the smallest fraction (0.1>10 μm) able can to access all organs, by crossing cell membranes, and passing into the blood-brain barrier and even the placenta [36,37]. There sizes of micro particles were found in different animal species of various compositions, ranging from (0.1-150 μm), including humans (size of particles: 0.2-150 μm), dogs (3-100 μm), rabbits (0.1-10 μm), and rodents (30-40 μm) [35].
The penetration of micro-plastic particles to animal cells and tissues
The penetration of micro-plastic depends on their size and surface charge [36]. Besides, behavioral changes in Reproductive dysfunction (reduced fecundity, reduced behavioural of offspring) may also occur in aquatic life [37]. As well as, increased the rate of mortality [38]. Some studies showed micro plastics in chicken giblets, gizzard, and feces [39]. Nano-plastic Particles (NPs) have an effect on the homeostasis of the hormonal system in the living penetrated cells, and the growth performance of organisms [40].
Micro-plastic Particles (MPs) in foods
Many pieces of evidence confirmed the presence of NPs in food products such as bread, jiggery, corn flakes and biscuits besides, the average content of micro-plastics reported in honey was 0.166 fibers/g and 0.009 fragments/g. In beer and table salts, micro-plastic content was between 0.007 and 0.68 particles/g. The contaminants can be in the organic or inorganic nature [41]. This salt may be used in the animal's diets. Chinese mussels contained the highest number of micro plastics which is nearly median value with 4 particles/g [42]. On the other hand, Seafood may provide approximately 20% of animal protein intake [42].
The role of micro bacteria in the intestine
Some experiments on the mice showed that the absence of the intestinal microbiota has a major impact on the infection of animals As well; the micro biome composition affects the physiological functions of organs, intestinal vascularization, tissue regeneration, bone homeostasis, metabolism, and animal behavior [43]. In addition, humans do not have enzymes that digest cellulose, xylenes, resistant starch, or inulin. Intestinal microbes are thought to ferment these carbohydrates to produce short-chain fatty acids [44]. These primary acids can be converted into secondary bile acids through the intestinal microbiota [44]. Bile acids suppress the overgrowth of bacteria in the gut and have anti-microbial effect in maintaining a healthy gut [45]. The microbiota functions with the host’s defenses and the immune system protect against pathogen colonization and invasion [46]. Moreover, such microbiota has essential metabolic functions, acting as a source of essential nutrients and vitamins, aiding in the extraction of energy and nutrients, short-chain fatty acids and amino acids, from food [47]. The human intestinal microbiota plays a key role in metabolic, physiological, nutritional and metabolic, physiological, nutritional and immunological processes [48]. Micro-plastic Particles (MPs) increase viscosity in the intestine. This leads to suppress the interactions of digestive enzymes with nutrients (slowing degradation) and decrease the absorption of glucose and other nutrients [49].
The roles of bacteria against nano plastic particles
There is a relationship between dietary fiber and microbiota. Microorganisms form a dense and active community in the rumen and comprise a diverse array of bacteria, archaic, protozoa and fungi, with a wide variety from metabolic cap short-chain fatty acids and amino acids, from food [50]. The human intestinal microbiota plays a key role in abilities [51]. Production and active systems of bacteria depend on the grains and fermentable carbohydrates. Bacteria are involved in the first steps of biomass breakdown in the rumen. As well, there is a link between non-digested food and passing time in intestines.
The insoluble fibers directly help the animals to get rid of the nano-plastic particles in feces in a short time [52]. Furthermore, micro bacteria have contributed to metabolism, immune system development [53]. Whereas, the main fermentative activity in the gut are SCFAs, in particular, acetate, propionate, and butyrate [54]. On the other hand, a low-fiber diet is a key driver of microbiome depletion and may cause a decline in gut microbiome diversity [55]. As well, the intake of high-fat and high-sucrose diet can lead to the extinction of several taxa of the gut microbiota. The produced metabolites like (SCFAs) have a protective role in colonic inflammation [56]. Furthermore, different dietary patterns can act a role to modulate the evolution of the intestinal microbiome [57]. Whereas, microbes contribute 1.5-2 kg of human total body weight [58]. The colon is the most densely populated organ from micro biota [59]. Bacterial diets are linked with several health benefits due to their capacity to degrade complex sugars and proteins into metabolizable short-chain fatty acids and decreased the effect of toxic nano-plastic in gut intestine tract.
The impact of bacteria on nutritional fibers
Dietary fibers contain insoluble and soluble fiber, galactic oligosaccharides or fructooligosaccharides [49]. Resistant starch decreases by 70% of the DNA damage [52]. A resistant starch enriched diet increases the numbers of bifid bacteria and Lactobacilli species. While it decreases coliforms and higher levels of SCFAs [60]. Dietary fiber is degraded by the gut microbiota, and bacterial fermentation end products in the colon provide animals with 5-20% of their total energy The gut microbiome is a highly heterogeneous population comprised 1014 bacteria representing 5000 species and 5 million genes (collectively known as the met genome) [61]. Butyrate is the preferred energy source of colonic mucosal cells. Thus, SCFA may improve the health of the gut [62]. It plays an essential role in the metabolism of undigested carbohydrates and the biosynthesis of vitamins [47]. The microbiota is essential in the development of the intestinal mucosa. Additionally, Dominant and prevalent species of gut bacteria, including SCFAs producers, appear to play a critical role in the initial degradation of complex plant-derived polysaccharides [63]. As well, the gut microbiota contributes to the production of vitamins K and B12, and foliate [64]. As well the production of insole derivatives (for example, γ-aminobutyric acid), which affect the levels of a brain-derived neurotropic factor in the central nervous system in the organisms [65].
The main role of dietary fiber in the diets
On a worldwide basis, corn and soybean meal are the main staples in the diet for pigs and poultry, providing most of the energy and nutrients needed. Alternative feedstuffs, wheat, rice bran. The fate of fiber in the colon largely depends on the colonic microbiota and the physio-chemical characteristics of fibers [66]. The type of dietary fiber affects the microbial composition of the gut lumen. For example, inulin, a polymer of fructose monomers present in onions, garlic and asparagus, stimulate the growth of Bifid bacteria whereas, it restricts the growth of potentially pathogenic bacteria E. coli, Salmonella, and Listeria [62].
The positive effect of dietary fiber on animal health
Dietary Fibers increased colonic fermentation and short-chain fatty acids production, fecal bulk/(laxation), and consequently stimulates gut health and prevents colon cancer, while DF reduces many other biochemical metabolites such as total and/or LDL, or both serum cholesterol levels, glycemia/ insulinemia, blood pressure, weight loss, reduction in adiposity, Increased satiety, Beneficial effect on mineral absorption [67]. As well, SCFAs work on maintaining mucosal homeostasis. SCFAs are volatile compounds with short half-lives and rapid metabolism [68]. As well, they constitute, approximately, 10% of the energy source in healthy people a 1% increase of microbial metabolic activity increases calorie input to the host, the body may gain 1 kg per year [49].
The key functions of dietary fibers
Dietary fiber plays an important role in colon cancer prevention. It also reduces the contact time of carcinogens within the intestinal lumen and promotes healthy gut microbiota, which modifies the host’s metabolism in various ways. The gut microbiota enhances bile acid DE conjugation, produce short-chain fatty acids, besides, the beneficial role to modulate material bioactive for inflammatory [64]. Furthermore, dietary fiber is not only a substrate for fermentation, but it is also a source of vitamins, minerals, and slowly digestible energy, bran fractions rich in minerals, vitamin B6, thiamine, folate and vitamin E [69]. Dietary fiber is associated with phytochemicals phenolics, carotenoids, lignin, beta-glycan and inulin [69]. Arabinoxylan, a constituent of hemicelluloses, is an important source of phenolic compounds that released in the colon during fermentation of complex fibers [69].
The impact of nano-or micro-plastic on the living organisms
The accumulation of microplastics (<5 mm) in the gut, results in starvation and malnourishment of animals and ultimately leading to death [70]. Furthermore, the microplastics have the potential to be transferred between trophic levels such as honey, salt, and, seafood [71]. The complex and dynamic bacterial community plays an important role in general health for organisms. Furthermore, there are a lot of concerns for Nano plastic on the trophic levels. An alarm prediction is that by 2050, the oceans will contain more plastics by mass than fish [72]. The fish will have a lot of Nano plastic in the tissues will affect the human and the animals; this may lead to lower reproduction, growth, and fitness, and the biodiversity of microbial communities. ΜPs in human and other mammals, and enter the food [73]. Penetration of plastic the biological barriers will lead to an increase in the mortality rates for organisms more than 30% [74]. Some studies showed the absorption rate in warm-blooded organisms from nano plastic is faster than cold-blooded organisms 30 times faster than in seawater.
For this reason, if we cannot decrease the ratios of NPs or MPs in the animal’s diets, the human health can be improved. For example, the consumption ratio of Nano plastic is 40 mg/ person/day [75]. Microbes have been shown to degrade many plastic materials [76]. There is concern that microplastics could have adverse health effects on humans as they move through the marine food web. Microplastics both absorb and give off chemicals and harmful pollutants. Plastic's ingredients or toxic chemicals absorbed by plastics may build up over time and stay in the environment. Bottled water from major brands like Aquafina, Nestle, and Dasani contains tiny plastic particles, and evidence suggests they're major contributors to microplastic trash heaps. Studies suggest disposable, plastic water bottles can harbor hundreds of tiny bits of plastic, and we're drinking them down with bottled H2O. The biggest sources of human exposure to microplastics likely come from airborne dust, drinking water (including treated tap water and bottled water) and seafood (shellfish in particular, because we eat the entire animal), Ranchman says.
The penetration of nano plastic particles the human cells
The human can get 74000 particles/person/per years, it means around 5 gram per week. As well, the micro plastic has an effect on the human organs such as (liver, spleen, gastrointestinal tract, stomach, skin, water and in the faces. Through eating, drinking and breathing, ingest at least 74,000 micro plastic particles every year. Another recent study estimated that people consume about 5 grams of plastic a week [20]. Micro plastics can cross the hardy membrane protecting the brain from many foreign bodies that get into the bloodstream. However, biphenyl exposure to reduced fertility in men and women, it has also been linked to a number of health issues, including nervous system problems, hearing loss and cancer [37]. Including various cancers, a weakened immune system, and, reproductive problems, and, morphological differences in the gastrointestinal tract. Furthermore, the whole structure of the gut and the digestion process [7]. where, the abundance of MPs showed a significant effect on the tissue weight and organs, with intestine containing the highest MP levels (9.2 items/g of tissue), surprisingly followed by foot, the stomach, gills, mantle, adductor muscle, gonads, and the visceral mass. On the other hand, there are many emerging threats posed by leaching of plastic is endocrine disruption potential, growth and, a vehicle for pathogen spreading is also addressed on the animals [56]. Uptake efficiency also depends on the combination of particle size, shape, and density that determine MP position in the water column and/or sediments, and hence their availability to animals. For example, the estimated daily MP ingestion rates range from a minimum of about 100 pieces for fish [31-33].
In addition to feeding habits, the mechanism of ingestion and the structure of digestive organs also affect the uptake of MPs [33]. It showed that in freshwater fish plastic items are likely to accumulate in intestines [20]. As well, microplastics may be also ingested indirectly through trophic transfer, whereby contaminated preys are consumed by predators [56]. In other studies, By monitoring the accumulation of PS MPs following a 7-days exposure to 5 μm and 20 μm size particles, we found the 5 μm MPs in fish gills, gut, and liver, while 20 μm MPs accumulated only in gills and gut [76]. So, that it means there is a possibility for smaller MPs to be transferred to the liver through the circulatory system. Thus, MP can lead to decrease offspring of living organisms by 41% and 18%, respectively, compared with control. Micro plastics present a physical threat; it acts as a vector for chemical transfer. As well, accumulate these particles lead to effects on Bioaccumulation. So, it is likely that both the lungs and GIT have been exposed to non-degradable [75]. Recently, there had been an increased dietary influx of non-degradable micro particles, approximately 40 mg/person/ day, primarily due to their inclusion as additives in processed. Furthermore, nano plastic may attractive the harmful bacteria for human pathogens such as strains of Vibrio spp [41]. Thus, these chemicals of micro plastic have been linked to a variety of health problems, including reproductive harm, organ problems and growth performance. In this review we present an overview of the different exposure routes for animals and humans. And, the potential effects of MPs and NPs on the food chains and human health and the relationship with dietary fiber.
Strategies To Protect The Nature Against Nano Or Micro-Plastics
Many strategies can be used to decline the harm effect of nanoplastics. The First one is mechanical protection and to avoid exposure of the plastic containers to sunlight and other decomposition factors. Fodder on farms and small production units, and use screeners to remove impurities and plastic parts in feed stores or loss during the unloading process. The second thing to do is the nutritional side. Reducing the using fishmeal in the diets of monogastric animals, this is because many studies have shown that fish waste contains a high percentage of plastic materials due to the direct contact of fish with plastic debris in the oceans and rivers. Besides, use other protein replacement to raise protein levels in diets except for fish meal. Specially, that comes from highly polluted regions. Finally, increasing the diet’s content of dietary fiber to be an important factor for disposing of plastic materials in the event of the loss of the abovementioned factors. The dietary fibers lead to Increasing the volume of stools, preventing the work of digestive enzymes on the plastic entry with food. Thus, they prevent or limited entry or penetration or nano-plastic in the body of the living organism. Thus, by these ways were limited and prevent the effect of Nano plastic on the gastrointestinal tract or growth performance of animals, and the production [77].
Micro plastic is more harmful to living organisms, that is through the gastrointestinal tract of birds had plastic fragments beside, MPS could be toxic to aquatic organisms, they do not only cause a physical injury in fish, they also, block the digestive tract, reduce growth rates, block enzyme production, induce oxidative stress and even affect reproduction. Some studies found the MP in the chickens (47 to 57) mg. the ratio of microplastic particles in some experiments (0.5-50) microgram, 1000 microgram / litre. Also, in commercial diets for that, we showed the effects of NPS and MPS on the organisms, for that we must focus more on reducing the risk of Nano-plastic particles of the organisms and the ratios of its presence in the diets and the amount of it on the animals. That is through using the dietary fiber in the diets, as well, we should avoid using the plastic as possible in the animals diet.
Ethics approval and consent to participate: Not applicable because this article does not contain any studies with human participants or animals performed by any of the authors.
Not applicable
Availability of data and materials not applicable
The authors declare no conflict of interest.
Development of new standardized of livestock and poultry production, 2017YFD0502001
All authors have read and approved the manuscript.
We are thankful to the Jilin Agricultural University for providing all fasciitis to accomplish this work.
Citation: Hussein S, Farouk MH, Hussein A, Hailong J (2021) The Role of Dietary Fiber and Microbiome Composition to Decrease the Deleterious Effects of Nano-Plastic in Monogastric Animals. J Prob Health. 9:229.
Received: 24-Dec-2020 Accepted: 07-Jan-2021 Published: 14-Jan-2021 , DOI: 10.35248/2329-8901.21.9.229
Copyright: © 2021 Hussein S. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.