
Journal of Clinical Chemistry and Laboratory Medicine
Open Access
ISSN: 2736-6588

ISSN: 2736-6588
Research Article - (2022)Volume 5, Issue 5
Purpose: Explore whether thymosin affects the expression of PD-L1 on the surface of tumor cells, which in turn affects the immunotherapy of triple-negative breast cancer.
Methods: After treating human breast cancer MDA-MB-231 cells with different concentrations of thymosin α1, the PD-L1 expression of the cells was tested for protein and gene. Through bioinformatics analysis, the target gene was screened out, and the signal pathway that thymosin α1 might participate in was further verified.
R After adding 130 μM and 160 μM of thymosin α1, the expression of PD-L1 in MDA-MB-231 cells was inhibited at 12 h, 24 h, 48 h and 96 h. The related pathways of genes down-regulated by Tα1 in KEGG are: Cancer pathway, basal cell epithelioma, apoptosis, Hippo and Wnt signaling pathway; immune pathway, such as rheumatoid arthritis, bacterial infection. After MSAB treatment of the experimental group, the expression of AXIN2 was up-regulated, and the expression of β-catenin in the cells was up-regulated. Compared with the cells in the untreated experimental group, PD-L1 was up-regulated. However, we found that β-catenin in the nucleus was not significantly up-regulated, but the expression of C-myc was up-regulated result.
Conclusion: Thymosin α1 down-regulates the PD-L1 expression of TNBC through the Wnt/β-catenin pathway, which may affect PD-L1-dependent TNBC immunotherapy.
Thymosin α1; TNBC; Treatment; Signaling pathway
Breast cancer is one of the more common malignant tumors in women, and triple-negative breast cancer accounts for 10.0% to 20.8% of all breast cancer pathologies [1]. Triple-Negative Breast Cancer (TNBC) refers to the results of the immunohistochemical examination of tumor tissue for Estrogen Receptor (ER), Progestogen Receptor (PR) and Human Epidermal Growth Factor Receptor 2 (HER2) are all negative breast cancers [2]. Triple-negative breast cancer has special biological behavior and clinical characteristics, and its prognosis is worse than other types of breast cancer [3]. Therefore, it is important to find new treatment strategies to improve the efficacy and prognosis of patients with triple-negative breast cancer.
Programmed cell death 1 (PDCD1, also known as PD-1) and programmed cell death 1 ligand 1 (PDCD1LG1, also known as PD-L1) have emerged in recent years A new type of immunotherapy [4], which mainly induces tumor cell apoptosis by blocking the PD-1/PD-L1 signaling pathway to achieve the therapeutic goal [5]. Relevant studies have shown that PD-1/PD-L1 targeted therapy has good prognostic results for advanced malignant melanoma [6].
Under normal circumstances, the human body eliminates pathogenic microorganisms and necrotic cells in the body through positive immune regulation and avoids autoimmune system diseases through negative regulation mechanisms. Negative regulatory costimulatory molecules are usually called immune checkpoints, and programmed death receptor 1 and its ligands are important ones. Immunosuppressive therapy cuts off the negative regulatory pathway, activates T cells, and activates the body's anti-tumor immune response system, thereby achieving the effect of targeted elimination of tumor cells. In TNBC, immune checkpoint PD-L1 inhibitor Pembrolizumab combined with chemotherapy drugs can significantly increase the pathological complete response rate in adjuvant breast cancer treatment. Therefore, inhibiting the binding of PD-1 and PD-L1 to avoid tumor immune escape is a potential target for the treatment of TNBC.
In a study, the immunohistochemical examination of the biopsy specimens of 42 patients was performed to evaluate the expression of PD-L1 on the surface of tumor cells. Among 17 PD-L1-negative patients, there was no objective response to the treatment of anti-PD-1. This data suggests that the expression of PD-L1 on the surface of tumor cells may be a tumor biomarker for evaluating the effectiveness of anti-PD-1 therapy [7]. In tumor therapy, thymosin mainly enhances the immune system function through the regulation of the immune system, improves the killing effect of immune cells on tumor cells, and at the same time enhances the anti-tumor activity, to achieve the purpose of treating tumors and resisting tumor metastasis. Finally, by improving the body's immunity, speeding up the recovery of immune function, improving immunosuppression, organ damage, and other negative effects in the process of anti-tumor treatment, and finally achieving the role of adjuvant therapy and improving prognosis. Therefore, we suspect that thymosin may affect the expression of PD-L1 on the surface of tumor cells and further affect the treatment of clinical tumors.
Material
Cell line Human breast cancer MDA-MB-231 cells were purchased from the Shanghai Chinese Academy of Sciences Cell Bank.
Cell culture
Resuscitate the cells: quickly shake the cryotube containing 1 mL of cell suspension in a 37℃ water bath to thaw, add 5 mL of medium and mix well. Centrifuge at 1000 RPM for 5 minutes, discard the supernatant, add 4-6 mL complete medium and blow well. Then add all the cell suspension to the culture flask and culture overnight (or add the cell suspension to a 6 cm dish), and culture overnight. Change the medium the next day and check the cell density. Cell passage: If the cell density reaches 80%-90%, it can be subcultured.
Cell processing and grouping
Compliance was calculated based on dispensed and returned study products. In the case of missing products or no return, compliance was estimated from subject diaries. After drug treatment in MDA-MB-231 cell line (0 μM, 40 μM, 70 μM, 100 μM, 130 μM, 160 μM, respectively, treatment time points 12 h, 24 h, 48 h, 96 h), 3 replicate wells for each treatment concentration; protein extraction WB detection and RNA extraction for Q-PCR detection. The cells treated with 160 uM were used as the observation object.
WB detection
Inoculate the MDA-MB-231 cells in the logarithmic growth phase in a culture flask. When the cells grow to 80%, discard the medium and add Thymosin α1 (0 μM, 40 μM, 70 μM, 100 μM, 130 μM, 160 μM) with different concentrations, and treat. The time is 12, 24, 48, 96 h. Wash 3 times with PBS buffer, add cell lysate (RIPA: PMSF: Cocktail=100:1:2) on ice for 30 min, 12 000 r•min-1 centrifugation for 20 min, use BCA method to detect protein concentration, add Load the protein buffer solution, place it in boiling water for high-temperature denaturation for 8 min, take 100 μg of protein, separate it by 10% SDS-polyacrylamide gel electrophoresis, transfer it to PVDF membrane, and block with 5% BSA at room temperature for 40 min. The antibody was incubated overnight on ice, and the secondary antibody was incubated at room temperature for 60 min. Finally, the membrane was scanned with a dual-color infrared fluorescence scanning imaging system and images were collected. Each experiment was repeated 3 times.
Q-PCR detection
Inoculate the MDA-MB231 cells in the logarithmic growth phase in a culture flask. When the cells grow to 80%, discard the culture medium and add thymosin α1 (0 μM, 40 μM, 70 μM, 100 μM, 130 μM, 160 μM) containing different concentrations of thymosin α1 (0 μM, 40 μM, 70 μM, 100 μM, 130 μM, 160 μM) for treatment. The time is 12, 24, 48, 96 h. Wash 3 times with PBS buffer, extract the total RNA of MDA-MB-231 cells with the RNA extraction kit (Axygen), measure the content and purity of the total RNA with a nucleic acid detector, and use the Thermo reverse transcription kit to reverse 1 μg of the total RNA Recorded as cDNA. Carry out real-time fluorescent quantitative polymerase chain reaction (qRT-PCR) with EGFR gene-specific primers, and operate according to the instructions. Reaction steps: 95℃ 10 min, 95℃ 15 s, 60℃ 1 min, a total of 40 cycles. Let 2-ΔΔCT represent the relative expression of the gene, and the internal reference gene is GAPDH.
Library preparation, sequencing, and data analysis
After the total RNA is processed to remove the DNA, the absorbance at 260 nm/280 nm (A260/A280) is measured to determine the quality and quantity of the purified RNA. 1.5% agarose gel electrophoresis further verified the integrity of RNA. Polyadenylated mRNAs are purified and concentrated with oligo (dT) bound magnetic beads (In vitro) before they are used in the preparation of directed RNA sequence libraries. The purified mRNAs are lysed in 95°C lysis buffer, the ends are repaired, and the 5'linker is ligated. Then RT primers containing 3'adaptor sequences and random hexamers were used for reverse transcription. Purify and amplify the cDNAs, purify the PCR products of 200~500 bps, quantify and store them at -80℃ for sequencing. Prepare following the manufacturer's instructions and use the Illumina HiSeq X Ten system for sequencing. The software we choose is edgeR for gene differential expression analysis. The analysis result uses Fold Change (FC) and False Discovery Rate (FDR) to determine whether a gene is differentially expressed. Significant difference expression evaluation criteria: FC ≥ 2 or ≤ 0.5, P-value <0.01.
Wnt/β-catenin signaling pathway blockade
After the Wnt/β-catenin signaling pathway inhibitor, MSAB was used to treat the cells in the experimental group; Western blot was used to detect the expression of AXIN2, β-catenin, PD-L1, and C-myc.
Statistical analysis
The experimental result data is expressed as x̅ ± s, and the SPSS 26.0 software was used for statistical analysis. The comparison of means between groups was performed by t-test, and the comparison of means above two groups was performed by one-way analysis of variance.
The effect of thymosin α1 on the expression of PD-L1 in TNBC
With the addition of thymosin α1 130 and 160 μM, the expression of PD-L1 was inhibited in the 12 h, 24 h, 48 h, and 96 h detections, and the inhibition was more significant with the addition of 160 Μm (Figures 1 and 2).
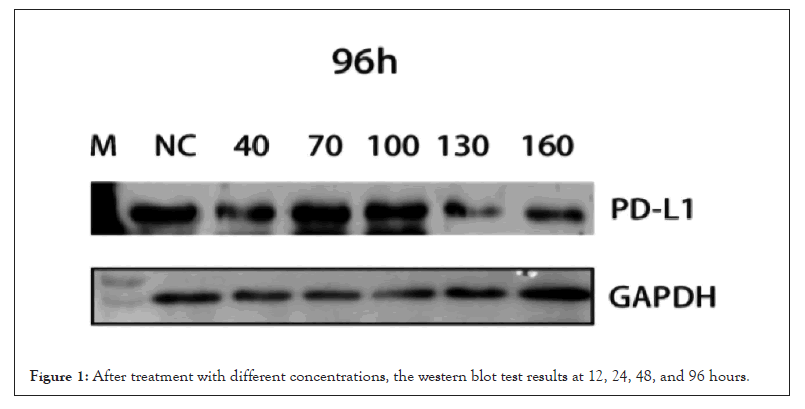
Figure 1: After treatment with different concentrations, the western blot test results at 12, 24, 48, and 96 hours.
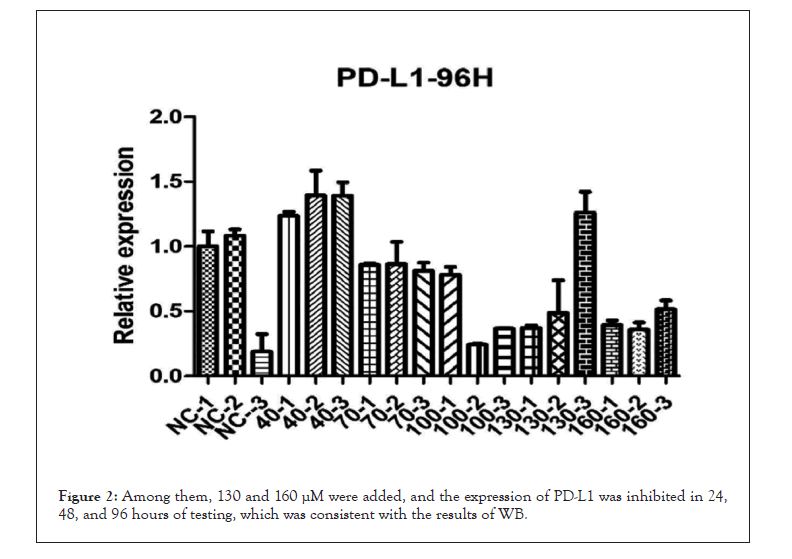
Figure 2: Among them, 130 and 160 μM were added, and the expression of PD-L1 was inhibited in 24, 48, and 96 hours of testing, which was consistent with the results of WB.
Transcriptome differential expression analysis results
Genes down-regulated by Tα1 is enriched in cancer-related pathways in KEGG, such as cancer pathway, basal cell epithelioma, apoptosis, Hippo and Wnt signaling pathways; immune pathways, such as rheumatoid arthritis, bacterial infections (Table 1 and Figure 3).
| Term | Input number | Background number | P-value | Corrected P-value | Input |
|---|---|---|---|---|---|
| Pathways in cancer | 16 | 397(5.63%) | 1.39E-05 | 0 | RASGRP2 EGLN3 PDGFB BDKRB2 AXIN2 WNT10A Bl |
| -19.05% | RC3 WNT4 COL4A6 FGF19 BMP4 GNG2 CXCL8 WN | ||||
| T5A WNT7B FGF14 | |||||
| Basal cell carcinoma | 6 (7.14%) | 55(0.78%) | 4.32E-05 | 0 | BMP4 AXIN2 WNT10A WNT4 WNT5A WNT7B |
| Hippo signaling pathway | 8 (9.52%) | 154(2.18%) | 0.000459 | 0 | AREG BMP5 BMP4 AXIN2 WNT10A WNT4 WNT5A W |
| NT7B | |||||
| Signaling pathways regulating pluripotency of stem cells | 7 (8.33%) | 142(2.01%) | 0.001434 | 0 | BMP4 AXIN2 WNT10A ID3 WNT4 WNT5A WNT7B |
| Rheumatoid arthritis | 5 (5.95%) | 91(1.29%) | 0.004425 | 0 | CCL2 LTB ANGPT1 ATP6V1G3 CXCL8 |
| Staphylococcus aureus infection | 4 (4.76%) | 57(0.81%) | 0.00457 | 0 | FPR1 C4A C4B CFH |
| Steroid hormone biosynthesis | 4 (4.76%) | 58(0.82%) | 0.004864 | 0 | CYP21A2 AKR1C1 SULT2B1 AKR1C3 |
| Melanogenesis | 5 (5.95%) | 100(1.42%) | 0.006593 | 0 | CREB3L3 WNT5A WNT4 WNT10A WNT7B |
| Apoptosis-multiple species | 3 (3.57%) | 33(0.47%) | 0.006862 | 0 | SEPT4 BIRC3 NGFR |
| Wnt signaling pathway | 6 (7.14%) | 143(2.03%) | 0.006935 | 0 | SOST AXIN2 WNT10A WNT4 WNT5A WNT7B |
Table 1: Genes down-regulated by Tα1 are enriched in cancer-related pathways in KEGG, such as cancer pathway, basal cell epithelioma, apoptosis, Hippo and Wnt signaling pathways; immune pathways, such as rheumatoid arthritis, bacterial infections.
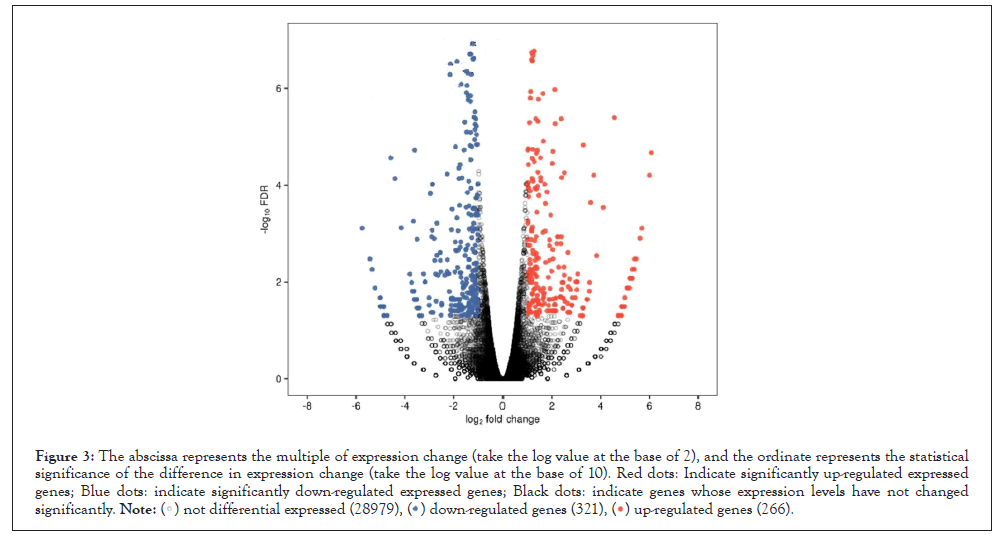
Figure 3: The abscissa represents the multiple of expression change (take the log value at the base of 2), and the ordinate represents the statistical
significance of the difference in expression change (take the log value at the base of 10). Red dots: Indicate significantly up-regulated expressed
genes; Blue dots: indicate significantly down-regulated expressed genes; Black dots: indicate genes whose expression levels have not changed
significantly. .
.
Expression of Wnt/β-catenin signaling pathway
After the cells in the experimental group were treated with MSAB, the expression of AXIN2 was up-regulated, the expression of β-catenin in the cells was up-regulated, and the expression of PD-L1 was up-regulated compared with the cells in the untreated experimental group. However, we found that β-catenin in the nucleus was not significantly up-regulated. But the expression of C-myc was up-regulated (Figure 4).
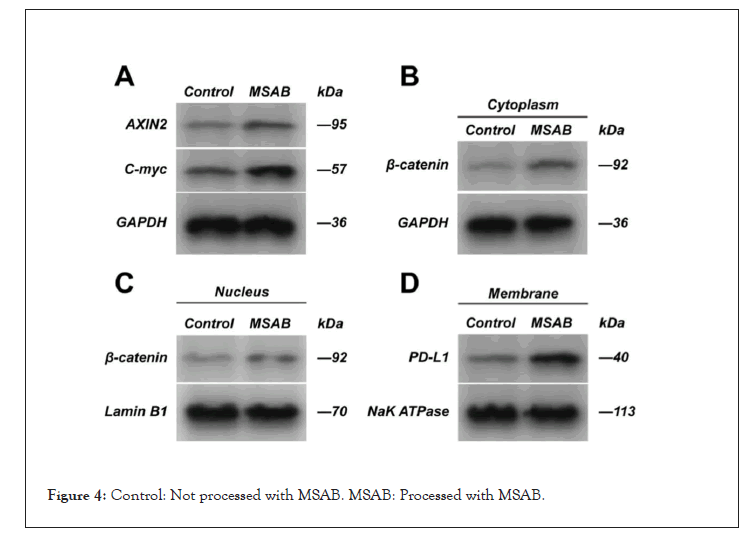
Figure 4: Control: Not processed with MSAB. MSAB: Processed with MSAB.
Thymosin α1 is a class of immunomodulators [8], which can transform T cells in the thymus into mature T lymphocytes, further differentiate into T cell subgroups with different functions and make them proliferate. In addition, related studies have shown that thymosin α1 also has the effect of controlling inflammation and regulating immune tolerance [9]. The immunomodulatory effect of thymosin enhances the body's cellular immune function [10], allowing it to directly or indirectly act on tumor cells and ultimately kill tumor cells. According to related studies, thymosin α1 can not only directly induce the apoptosis of kidney cancer cells, but also inhibit the growth of lung cancer and gastric cancer cells. Thymosin α1 is applied to the treatment of tumors. On the one hand, thymosin α1 significantly improves the anti-tumor function of T cells, normalizes the disordered immune function in the patient's body, and exerts an immune surveillance effect on tumors. On the other hand, it enhances the body's low anti-infective power caused by the effects of tumors and radiotherapy and chemotherapy drugs. Rasi, et al. [11] believe that thymosin α1 can increase the number of white blood cells in tumor patients, and ultimately increase the activity of NK cells that were originally suppressed. Relevant studies have shown that thymosin α1 is used to treat breast cancer patients. After comparative treatment, the NK cell activity of patients is significantly increased compared with that before treatment [12], which indicates that thymosin α1 has a certain adjuvant therapy effect on tumor patients. Our research found that thymosin α1 can down-regulate the expression of PD-L1 in triple-negative breast cancer cells, thereby reducing the binding of PD-1 molecules on the surface of tumor-infiltrating lymphocytes, and improving the tumor-killing function of lymphocytes. And through transcriptome analysis technology, we found that the genes that are up-regulated or down-regulated by thymosin α1 include cancer-related pathways and immune pathways. So we speculate that adenopeptide α1 may have an impact on immunotherapy. Further studies have found that adenopeptide α1 can down-regulate the expression of PD-L1 in triple-negative breast cancer cells through the Wnt/β-catenin pathway. This process may be achieved through the down-regulation of the expression of AXIN2.
The lack of therapeutic targets for triple-negative breast cancer makes its treatment challenging. PD-L1 is almost undetectable in normal breast tissue, but it is as high as 30% in triple-negative breast cancer, suggesting that immune checkpoint suppression may be a useful treatment. Compared with other types of breast cancer, TNBC has higher expression levels of tumor-infiltrating lymphocytes and PD-L1 in the tumor microenvironment of TNBC [13]. On the one hand, tumor-infiltrating lymphocytes can predict the prognosis and efficacy of TNBC patients. On the other hand, TNBC is more likely to benefit from the treatment of immune checkpoint inhibitors targeting PD-1/PD-L1. Studies have shown that about 20% of TNBC patients have positive PD-L1 expression, which is significantly higher than that of non-TNBC patients [14]. However, the specific mechanism of PD-L1 upregulation in TNBC is not yet clear. Mittendorf, et al. [15] found that the expression of PD-L1 may be related to the loss of PTEN and the activation of the PIK3CA pathway. The up-regulated PD-L1 binds to PD-1 on the surface of tumor-specific CD8+ T cells, thereby inhibiting the host immune response. In addition, inflammatory factors such as Interferon-γ (IFN-γ) in the tumor microenvironment can also induce the expression of PD-L1 and PD-L2, and achieve self-protection through "adaptive immune resistance" [16]. PD1/PD-L1/2 pathway mediates "tumor-specific T cell-selective suppression", therefore, through PD-1 immune checkpoint inhibitors, tumor-infiltrating CD8+ T cells that can recognize tumor neoantigens can be expanded, restore T cell function.
An important aspect of immunotherapy is to explore biomarkers that can monitor tumor-specific immune responses. PD-L1, TILs, tumor mutational burden, etc. have been proven to be effective markers, among which PD-L1 is one of the first biomarkers to be applied. However, some studies have shown that some PD-L1 negative people also have a certain response to PD-1 inhibitors. Therefore, while carefully interpreting the PD-L1 test results, more in-depth research should be carried out to comprehensively and accurately evaluate the predictive effect of PD-L1. A recent meta-analysis of patients with malignant melanoma or NSCLC showed that PD-L1 expression is significantly related to the response to PD-1/PD-L1 guided therapy [17]. Nevertheless, PD-L1-negative patients may still have a blocking response to PD-1. Therefore, careful consideration should be given to assessing PD-L1 expression to identify whether patients receive PD-1/PD-L1 guided therapy. Studies have found that in the process of immunotherapy, although PD-L1 expressed by tumors is an important factor in promoting tumor occurrence and development, in the tumor microenvironment, detecting the total amount of PD-L1 expression can more accurately determine PD-L1 the efficacy of the drug. This shows that PD-L1 expressed by the immune system also plays a role in the process of tumor immune escape.
Medical research believes that the process of tumor occurrence and development is directly related to the body's immune system. Only when the body's cellular immune function is in a normal state, can the body's defense against tumor cells be maintained. In the process of the interaction between the body's immune system and tumor cells, once the body's immune function loses its ability to regulate the excessive proliferation of tumor cells, the body's immune function will continue to decline at this time, and the disorder of the immune function will be counterproductive. Tumors make the condition worse. In this process, T lymphocytes are the protagonist of the battle. When the body's immunity is suppressed, the numbers of CD3, CD4, and CD8 T cells show a continuous decrease. It is very important and necessary to use immune enhancers to enhance the body's immune function in the treatment of tumors [18].
However, in the era of precision oncology, predictors of the effects of immune checkpoint therapy are crucial for patients who benefit from PD-L1 guided therapy. From our research results, after treatment with thymosin α1, the expression of PD-L1 in three-cause breast cancer cells will significantly decrease, which may affect the therapeutic effect of PD-L1 inhibitors. Thymosin α1 down-regulates the PD-L1 expression of TNBC through the Wnt/β-catenin pathway, which may affect PD-L1-dependent TNBC immunotherapy.
Not Applicable.
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript. That the article is original, has not already been published in a journal, and is not currently under consideration by another journal.
The data that support the findings of this study are available from the corresponding author, [Jian Zheng], upon reasonable request.
We declare that we have no financial and personal relationships with other people or organizations that can inappropriately influence our work, there is no professional or other personal interest of any nature or kind in any product, service and/or company that could be construed as influencing the position presented in, or the review of, the manuscript entitled, “The effect of immunomodulator thymosin α1 on the expression of TNBC PD-L1 through Wnt/β-catenin signaling pathway”.
Not Applicable
Guarantor of integrity of the entire study:Jian Zheng
Study concepts:Jian Zheng
Study design:Xiaoxi Li
Data analysis:Jian Zheng
Statistical analysis:Xiaoxi Li
Manuscript preparation: Youhong Jiang
Manuscript editing:Youhong Jiang
Manuscript review:Cunwei Cai
[Crossref][Google Scholar][PubMed].
[Google Scholar][PubMed].
[Google Scholar][PubMed].
[Crossref][Google Scholar][PubMed].
Citation: Jian Z, Xiaoxi L, Youhong J, Cunwei C (2022) The Effect of Immunomodulator Thymosin α1 on the Expression of TNBC PD-L1 through Wnt/β-Catenin Signaling Pathway. J Clin Chem Lab Med. 5:223.
Received: 17-May-2022, Manuscript No. JCCLM-22-17525; Editor assigned: 20-May-2022, Pre QC No. JCCLM-22-17525 (PQ); Reviewed: 06-Jun-2022, QC No. JCCLM-22-17525; Revised: 13-Jun-2022, Manuscript No. JCCLM-22-17525 (R); Published: 20-Jun-2022 , DOI: 10.35248/JCCLM.22.05.223
Copyright: © 2022 Jian Z, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.