Translational Medicine
Open Access
ISSN: 2161-1025
ISSN: 2161-1025
Research Article - (2025)Volume 15, Issue 1
Worldwide, an estimated 19.3 million new cancer cases (18.1 million excluding nonmelanoma skin cancer) and almost 10.0 million cancer deaths (9.9 million excluding nonmelanoma skin cancer) occurred in 2020. The global cancer burden is expected to be 28.4 million cases in 2040, Although there are several unique advantages for cancer treatment, in recent years, problems such as poor drug targeting efficacy, increased tumor hypoxia, severe coronary syndromes, excessive ventricular conduction and drug-induced drug resistance have emerged chemotherapy and increased risk of tumor metastasis have limited their potential clinical use. To deal with these challenges, it is vital to use compounds with the advantages of targeting molecules that are active in processes such as cell cycle, metastasis, angiogenesis, apoptosis and autophagy in the creation of a cancer mass. Hence the use of plant seed extract compound Silybum marianum which contains silymarin and polyphenolic derivatives due to its anti-inflammatory, immune system modulating, peroxidative, anti-lipid, anti-fibrotic, anti-oxidative and anti-proliferative activity, in addition to its anti-cancer functions, it has a protective role against chemotherapy-induced toxicity, such as toxicity kidney, liver plays an effective role in the treatment of cancer.
Angiogenesis; Chorioallantoic membrane; Silybum marianum; Cancer; Chemotherapy
CAM: Chorioallantoic membrane; Sy-A: Density 200 μg/ml; Sy-B: Density 400 μg/ml; Sy-C: Density 600 μg/ml; Ctr: Control group; VEGF: Vascular Endothelial Growth Factors; NF-κB: Nuclear Factor kappa-lightchain- enhancer of activated B cells; PI3K: Phosphoinositide 3-Kinases; MAPK: Mitogen-Activated Protein Kinases; STAT3: Signal Transducer and Activator of Transcription 3
Background
Worldwide, an estimated 19.3 million new cancer cases (18.1 million excluding nonmelanoma skin cancer) and almost 10.0 million cancer deaths (9.9 million excluding nonmelanoma skin cancer) occurred in 2020. Female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer. The global cancer burden is expected to be 28.4 million cases in 2040, a 47% rise from 2020. Efforts to build a sustainable infrastructure for the dissemination of cancer prevention measures and provision of cancer care in transitioning countries is critical for global cancer control [1].
Despite significant advancements in recent decades, cancer treatment still faces many challenges. The lack of effective therapeutic options and drug resistance are the main obstacles in cancer therapy. Thus, the cancer therapy area remained an unmet need which attracted scientists' attention to discover novel therapeutic components and strategies with minimal toxicity and higher efficacy.
Silymarin, derived from milk thistle (Silybum marianum) seeds, has been used as a natural remedy for more than 2000 years to target cirrhosis and hepatitis to protect the liver from toxic materials owing to its immunomodulatory, anti-inflammatory, anti-fibrotic, anti-lipid peroxidative and anti-oxidative properties. Silymarin could regulate the cell cycle, inhibit tumor growth and metastasis, induce apoptosis, regulate immune responses against tumor cells, and suppress angiogenesis. Mechanistically, the tumoricidal activities of silymarin are associated with its modulatory effects on several cell signaling pathways, including MAPK, PI3K/Akt, Wnt/β-Catenin, STAT3 and NF-κB [2].
Chemistry
The standardized extract obtained from the seeds of Sylibum marianum is known as silymarin which contains between 70% and 80% of silymarin flavolignans. Sylibum marianum is a mixture of 8 flavolignan structurally related isomers: Silybin (or silibinin), isosilibinin, silydianin, silychristin, isosilychristin and taxifolin. The main component of silymarin is silibinin which is a compound consisting of equal amounts of silybin A and silybin B.
Silybin is stable in acidic conditions but unstable under alkaline conditions. Alkaline media disrupt flavolignan's skeleton. This is important because the extracellular matrix of tumors has a low pH (approximate pH=6.8). Normal cells, on the other hand, have an alkaline extracellular milieu (approximate pH=7.35). We presume, without evidence to sustain the presumption, that silybin can reach the malignant cell's acidic extracellular space without degradation. This singular feature, the acidic extracellular pH of tumors, may explain why silybin effects differ in normal versus malignant cells. Silymarin may be able to better access the malignant cell compared with normal cells [3].
The chick Chorioallantoic Membrane (CAM) assay which shows the developing vascular net which supports the chick in the egg is one of the most commonly used in vivo assays for angiogenesis. This assay is widely used to screen pro-antiangiogenic drugs. As the CAM assay allows the direct visualization and quantification of sprouting angiogenesis in vivo without needing to sacrifice animals it has becoming a favourite assay for vascular biology, drug and vaccines development and cancer research.
Objectives
Previous studies have shown that the angiogenic system is involved in the growth of tumors whose size is estimated to be 2 mm. Also, no studies have been conducted to find medicinal compounds on the capillary branches that are moving from the main vessel to the tumor mass. Therefore, the purpose of this study is to investigate the natural medicinal compounds, including the compounds found in the hydroalcoholic extract of Silybum marianum plant [4].
Cell culture
7-MCF cell lines were obtained from the Shiraz medical sciences cancer research center cell bank and were cultured at a density of 2410 × 2 cells per square centimeter with RPMI1640 culture medium, FBS and Penstrep antibiotic in a 25 T flask. The cells were incubated at 37°C, 95% humidity and 5% carbon dioxide.
Extraction from Silybum marianum seeds
To extract the flavonolignans from the extract, first oil extraction was done from the seeds and the oil extraction method was based on the European Pharmacopoeia. The whole seeds of Silybum marianum were weighed, then milled and entered into the Soxhlet system. The plant material was defatted with N-hexane solvent for six hours. The amount of separated oil was weighed after complete removal of the solvent. After separating the oil, the seed powder was removed from the cartridge and completely dried for 24 hours under suitable conditions [5].
Finally, for 16 hours, using 70% methanol to extract flavonolignans, they were placed in a percolator apparatus. In the next step, in order to remove the solvent from the obtained extracts, we put them on a bain-marie with a temperature of 51 to 47 degrees celsius. Finally, we transferred the obtained dried golden powder to a container. In order to preserve the polyphenolic compounds in the extract until the day of the experiment, we put them in a cover of aluminum foil and kept them in the freezer at -18 degrees celsius.
MTT test
MTT assays were performed to evaluate the changes in the metabolic activities of the cells that occurred as a result of the hydroalcoholic extract treatment of Silybum marianum. The fibroblast cells planted in the wells were incubated for 24 hours. Then MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (Sigma-Aldrich, St. Louis, MO, USA) was added from the stock solution (5 mg L/L) was added to the cells in a 96-well plate (TPP) 24, 48 and 72 hours after treating the cells with Silybum marianum hydroalcoholic extract. The reaction was stopped after 4 hours of incubation at 37°C and the insoluble formazan was dissolved by adding Sodium Dodecyl Sulfate (SDS) to a final concentration of 3.3%. Absorbance (λ=584 nm) was measured using a BMG FLUOstar Optima (BMG Labtech GmbH, Offenburg, Germany). The results were evaluated as the absorption percentage of the untreated control [6].
Preparation of cell suspension
This suspension must be completed before implantation, which is done between days E 7-10 of the embryonated eggs (this procedure is the same for all cancer types). Extracellular matrix solution (Irana hydrogel matrix) was prepared on ice. Then the cells are suspended by trypsin and centrifuged at 1800 rpm-3000 rpm for 5-10 minutes and the supernatant is removed. Then the culture medium solution was added to the precipitated cell mass and pipetted slowly. We calculated the implantation volume in such a way that 1.2 × 106 cell/μl cells are implanted with a final extracellular matrix concentration of 201-204 mg/ml proteins. Then we put the cell-containing hydrogels in an incubator for 24 hours at a temperature of 37 degrees celsius and a humidity of 95% and 5% CO2.
Planting using hydrogel
We place the 7-day-old eggs on the egg rack in the biological cabin (choose a controllable number to prevent it from getting cold). We create a small hole with a punch machine on the air cavity and then on the shell. Finally, in the area where the false air cavity is created on the choroallantoic, a 1 × 1 valve is created and the cell suspension prepared from the previous step is placed in the center of the opened valve [7].
The opening is closed with a transparent film dressing and the eggs are returned horizontally without moving to the incubator with a humidity of 50%-60% and a temperature of 37-38 degrees celsius.
Drug injection
After 96 hours (E11), the samples were checked for cell viability and mass and then 200, 400 and 600 μg/ml of the drug prepared from Silybum marianum thistle extract, amounting to 20 units of insulin, were injected around the tumor area and then the valves were covered with a transparent dressing and returned to the device until the 14th day.
After 120 hours (E16) of the effect of the drug on the membrane, the eggs were placed in a freezer at -18 for 24 hours and in the last step, we imaged the mammospheres with a light microscope at x10 magnification. Then, the volume of mammospheres was measured with image j software and their growth graph was drawn with SPSS software. Based on this, the volume of mammospheres was obtained from the following formula, where W is the width, L is the length of the tumor.
Histology
Samples were fixed in Phosphate-Buffered Saline (PBS) 4% formaldehyde solution, paraffin embedded and cut into 5 μm-10 μm sections. These were stained by immunohistochemistry using antibodies specific for Ki67 (biotech). Negative controls were performed by omitting the primary antibody. Absolute numbers and percentages of positive cells in 13-28 regions of interest per experimental group were determined. Exemplary evaluation by a second examiner yielded similar results [8].
MTT
The survival rate of fibroblastic cells in concentrations of 400 μg/ml to 2000 μg/ml in 24 hours under treatment decreased significantly, also the percentage of survival in the concentration range of 600 μg/ml to 2000 μg/ml during 48 and 72 hours significantly decreased found (P 0.001). During 24 hours of treatment, the highest survival rate was observed at the concentration of 200 μg/ml (98.54%), while the lowest survival rate was observed at the concentration of 2000 μg/ml (25.11%) during 72 hours of treatment. IC50 level in fetal fibroblastic cell line in MTT was measured at 792.1 μg/ml in 24 hours by SPSS software (Figure 1) [9].
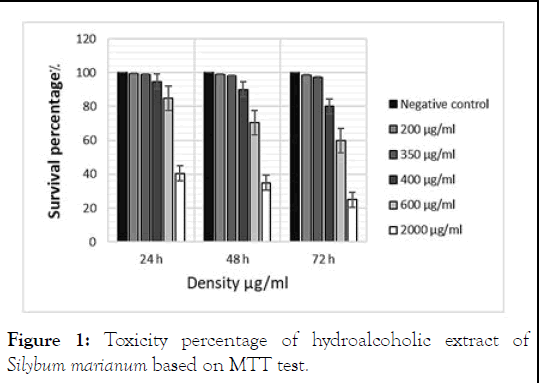
Figure 1: Toxicity percentage of hydroalcoholic extract of Silybum marianum based on MTT test.
Analysis of tumor size relative to vessel diameter
MCF-7 cells cultured in T75 flasks were well engrafted on the CAM membrane. 72 hours after tumor grafting to CAM (E-9), the grafted tumors were at a rate of 95%. 48 hours after treating the chorioallantoic region with the hydroalcoholic extract of Silybum marianum (E-11), the samples were checked for viability and returned to the incubator.
The average vessel diameter and tumor volume in experimental group 1 was compared to the control group (1.38 ± 0.98), which showed a significant decrease (p=0.001). Calculations for examining the average vessel diameter and tumor volume in experimental group 2 compared to the control group (1.07 ± 0.12) showed a significant decrease (p=0.0001). This is while in the experimental group 3, the decrease in the diameter of the vessels with the volume of the tumor (0.46 ± 0.25) and showed a significant decrease (p=0.0001). Also, to confirm the theory, the area of the tumor mass was also calculated.
Based on the obtained data, the average diameter of the vessels and the area of the transplanted tumor mass in the experimental group 1 compared to the control group (1.61 ± 0.08), which is a significant decrease You have (p=0.001) showed also, the average diameter of the vessels and the ratio to the area of the tumor mass were calculated for the experimental group 2 (1.39 ± 0.10) and these calculations for the experimental group 3 (0.73 ± 0.31) with a significant decrease (p=0.0001) was (Figure 2) [10].
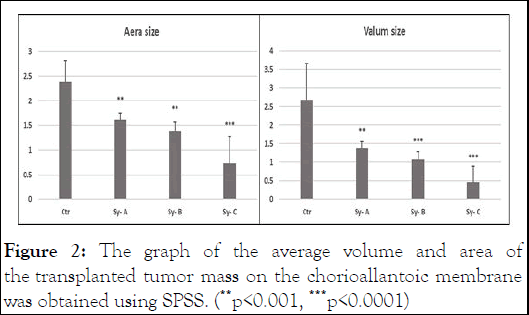
Figure 2: The graph of the average volume and area of the transplanted tumor mass on the chorioallantoic membrane was obtained using SPSS. (**p<0.001, ***p<0.0001)
Based on the analysis of the images by Image J software and the acquisition of data by SPSS (version 26) software, it was shown that with the decrease in the diameter of the vessels, the number of branching vessels that went towards the tumor graft mass decreased. As a result, a hypoxia in the tumor area and ischemia and apoptosis occur in the cells and the size of the tumor mass decreases (Figure 3).
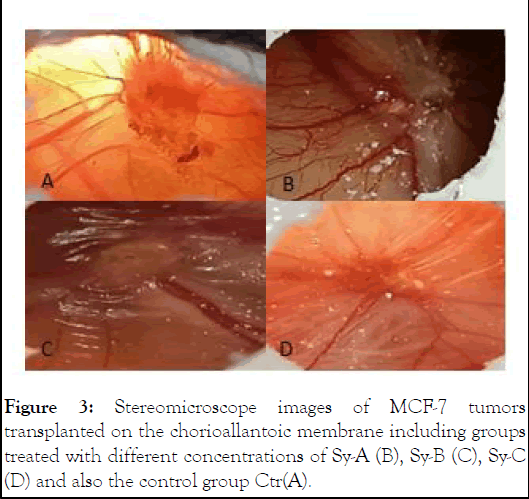
Figure 3: Stereomicroscope images of MCF-7 tumors transplanted on the chorioallantoic membrane including groups treated with different concentrations of Sy-A (B), Sy-B (C), Sy-C (D) and also the control group Ctr(A).
To better determine the difference in the size of transplanted tumors, showing the number of branches and vessel diameter in the control group and experimental groups, an analysis was performed by Image J software, which shows the amount of branched vessels towards the tumor mass in the chorioallantoic membrane in the control group and it shows the experimental groups (Figure 4).
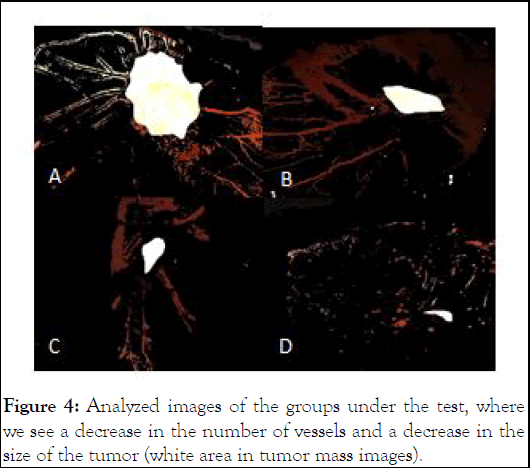
Figure 4: Analyzed images of the groups under the test, where we see a decrease in the number of vessels and a decrease in the size of the tumor (white area in tumor mass images).
Test immunohistochemical
Ki-67 proliferative marker was observed in 18 of the 24 tested samples. The degree of staining was one positive (*) in 18 cases and three positive (***) in 6 cases. Between tumor stage and proliferative biomarker expression Ki67 there is a significant relationship (Figures 5-8).
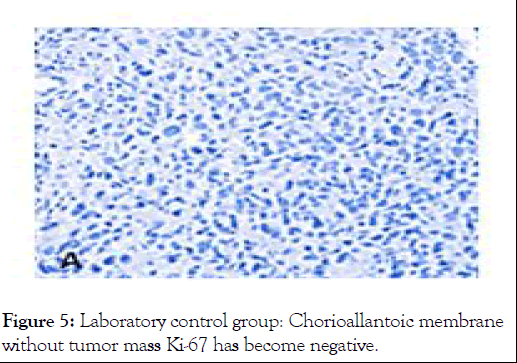
Figure 5: Laboratory control group: Chorioallantoic membrane without tumor mass Ki-67 has become negative.
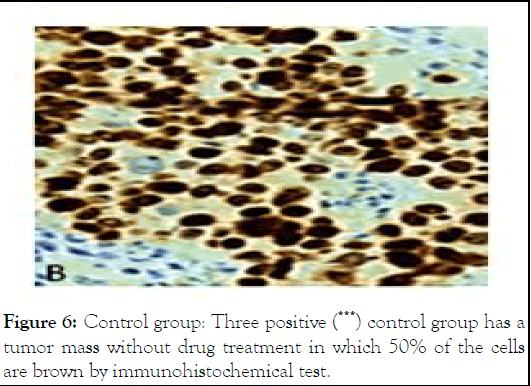
Figure 6: Control group: Three positive (***) control group has a tumor mass without drug treatment in which 50% of the cells are brown by immunohistochemical test.
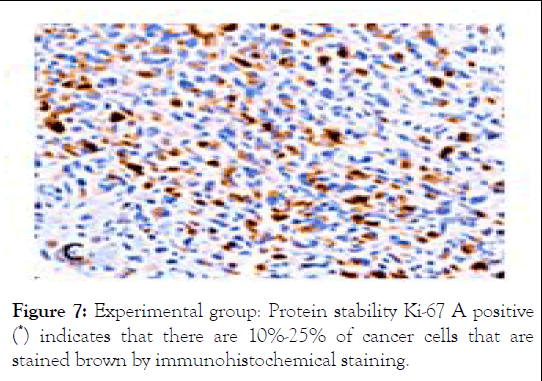
Figure 7: Experimental group: Protein stability Ki-67 A positive (*) indicates that there are 10%-25% of cancer cells that are stained brown by immunohistochemical staining.
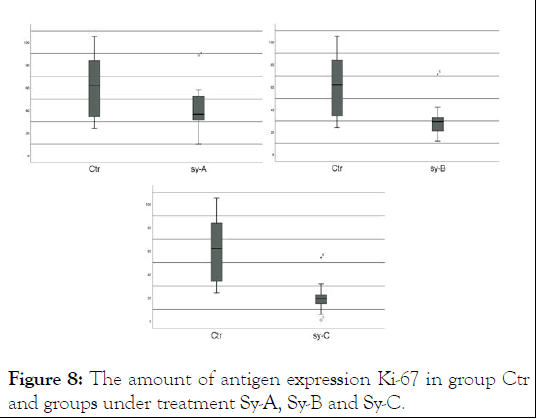
Figure 8: The amount of antigen expression Ki-67 in group Ctr and groups under treatment Sy-A, Sy-B and Sy-C.
Silymarin exerts its anti-cancer effects through different molecular pathways, including anti-angiogenesis and apoptosis pathways. This function of silymarin in Silybum marianum extract allows its use as a preventive and therapeutic agent for advanced and aggressive types of cancer. According to this Sung-Hyun et al. showed that the proliferation of MDA-MB-231 and MCF-7 breast cancer cells was inhibited depending on the concentration of silymarin.
Fan et al., reported a concentration-dependent decrease in ovarian cancer cells treated with different silymarin concentrations, where the effect was observed from 50 μg/ml. Deep et al., observed a concentration and time-dependent decrease in the cell viability rate of PC-3 prostate cancer cells when treated with 50 μg/ml and 100 μg/ml silymarin. Additionally, Kalla et al., demonstrated that the viability and proliferation of breast cancer cells were inhibited by different concentrations of silymarin. Vaid et al., also observed that the viability rates of melanoma cells were inhibited in a concentration-dependent manner when treated with different concentrations of silymarin.
Based on the findings Liang Yu at al. reported that silymarin can inhibit cell proliferation due to its ability to reduce the accumulation of β-catenin and cyclin D1. In support of these findings, Fan et al., reported apoptotic bodies in ovarian cancer cells treated with different concentrations of silymarin. Furthermore, Katiyar et al. revealed that apoptotic bodies in the skin epidermal cells were increased in a concentration-dependent manner following silymarin treatment. To summarize, the decrease in the viability of silymarin-treated MDA-MB-231 and MCF-7 breast cancer cells was closely related with the induction of apoptosis. According to the findings, the cause of apoptosis in cancer cells is due to the stopping of the angiogenic pathway in the growth process of tumor cells, on the basis of which Miyazawa M et al. reported that silymarin suppressed HIF-1α expression under hypoxic conditions in ovarian clear-cell carcinoma cells. Lin CH et al., reported that silymarin pretreatment inhibited HIF-1α subunit accumulation, as well as VEGF secretion in retinal pigmented epithelial cells.
Singh RP et al., reported the antiproliferative and proapoptotic effects of silymarin on human colorectal carcinoma HT29 cell were associated with down-regulated HIF-1α and VEGF expressions. Therefore, we studied the effect of the hydroalcoholic extract of Silybum marianum, which contains high amounts of silymarin, on the tumor grafted on the chorioallantoic of chicken embryos.
Our results showed that a specific concentration (200, 400 and 600 μg/ml) of the extract prepared from the seeds of the Silybum marianum plant on angiogenesis in the chorioallantoic has an inhibitory effect and reduces the growth of the transplanted tumor mass on the chorioallantoic membrane. In conclusion, our results provided evidence that silymarin exerts its antitumor effects through inhibiting the HIF-1α/VEGF axis under hypoxia in the MCF-7 cell line, which could be a potential silymarinbased drug therapy for cancer.
No funding was received for conducting this study.
The authors declare that there is no conflict of interest.
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
[Crossref] [Google Scholar] [PubMed]
Citation: Rouygar F (2025) The Effect of Hydroalcoholic Extract of Silybum marianum on Tumor Angiogenesis. Trans Med. 15:343.
Received: 06-Apr-2024, Manuscript No. TMCR-24-30693; Editor assigned: 11-Apr-2024, Pre QC No. TMCR-24-30693 (PQ); Reviewed: 25-Apr-2024, QC No. TMCR-24-30693; Revised: 03-Apr-2025, Manuscript No. TMCR-24-30693 (R); Published: 10-Apr-2025 , DOI: 10.35248/2161-1025.25.15.343
Copyright: © 2025 Rouygar F. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.